Proteasome inhibition reduces avian reovirus replication and apoptosis induction in cultured cells
- PMID: 18455810
- PMCID: PMC7119659
- DOI: 10.1016/j.jviromet.2008.03.016
Proteasome inhibition reduces avian reovirus replication and apoptosis induction in cultured cells
Abstract
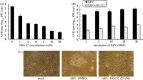
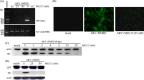
The interplay between avian reovirus (ARV) replication and apoptosis and proteasome pathway was studied in cultured cells. It is shown that inhibition of the proteasome did not affect viral entry and host cell translation but had influence on ARV replication and ARV-induced apoptosis. Evidence is provided to demonstrate that ubiquitin-proteasome blocked ARV replication at an early step in viral life cycle. However, viral transcription and protein translation were also reduced markedly after addition of proteasome inhibitor MG132. Treatment of BHK-21 cells with the MG132 markedly decreased virus titer as well as prevented virus-induced apoptosis. The expression of ARV proteins sigmaC, sigmaA, and sigmaNS was also reduced markedly, suggesting that suppression of virus replication is due to down-regulation of these ARV proteins by ubiquitin-proteasome system. MG132 was also shown to suppress ARV sigmaC-induced phosphrylation of p53 on serine 46, caspase 3 activities, and DNA fragmentation leading to complete inhibition of ARV-induced apoptosis.
Figures


Similar articles
-
p17-Modulated Hsp90/Cdc37 Complex Governs Oncolytic Avian Reovirus Replication by Chaperoning p17, Which Promotes Viral Protein Synthesis and Accumulation of Viral Proteins σC and σA in Viral Factories.J Virol. 2022 Mar 23;96(6):e0007422. doi: 10.1128/jvi.00074-22. Epub 2022 Feb 2. J Virol. 2022. PMID: 35107368 Free PMC article.
-
Molecular chaperone TRiC governs avian reovirus replication by protecting outer-capsid protein σC and inner core protein σA and non-structural protein σNS from ubiquitin- proteasome degradation.Vet Microbiol. 2022 Jan;264:109277. doi: 10.1016/j.vetmic.2021.109277. Epub 2021 Nov 10. Vet Microbiol. 2022. PMID: 34826648
-
Apoptosis induction by avian reovirus through p53 and mitochondria-mediated pathway.Biochem Biophys Res Commun. 2007 May 11;356(3):529-35. doi: 10.1016/j.bbrc.2007.02.164. Epub 2007 Mar 12. Biochem Biophys Res Commun. 2007. PMID: 17379188
-
Induction and attenuation of neuronal apoptosis by proteasome inhibitors in murine cortical cell cultures.J Neurochem. 2005 Nov;95(3):684-94. doi: 10.1111/j.1471-4159.2005.03393.x. Epub 2005 Sep 2. J Neurochem. 2005. PMID: 16144541
-
Avian reovirus sigmaC protein induces apoptosis in cultured cells.Virology. 2004 Mar 30;321(1):65-74. doi: 10.1016/j.virol.2003.12.004. Virology. 2004. PMID: 15033566
Cited by
-
Avian Reovirus Protein p17 Functions as a Nucleoporin Tpr Suppressor Leading to Activation of p53, p21 and PTEN and Inactivation of PI3K/AKT/mTOR and ERK Signaling Pathways.PLoS One. 2015 Aug 5;10(8):e0133699. doi: 10.1371/journal.pone.0133699. eCollection 2015. PLoS One. 2015. PMID: 26244501 Free PMC article.
-
Rotavirus replication requires a functional proteasome for effective assembly of viroplasms.J Virol. 2011 Mar;85(6):2781-92. doi: 10.1128/JVI.01631-10. Epub 2011 Jan 12. J Virol. 2011. PMID: 21228236 Free PMC article.
-
The transient nature of Bunyamwera orthobunyavirus NSs protein expression: effects of increased stability of NSs protein on virus replication.PLoS One. 2013 May 8;8(5):e64137. doi: 10.1371/journal.pone.0064137. Print 2013. PLoS One. 2013. PMID: 23667701 Free PMC article.
-
Viral Proteins as Emerging Cancer Therapeutics.Cancers (Basel). 2021 May 3;13(9):2199. doi: 10.3390/cancers13092199. Cancers (Basel). 2021. PMID: 34063663 Free PMC article. Review.
-
The ubiquitin-proteasome system is necessary for the replication of duck Tembusu virus.Microb Pathog. 2019 Jul;132:362-368. doi: 10.1016/j.micpath.2019.04.044. Epub 2019 May 1. Microb Pathog. 2019. PMID: 31054366 Free PMC article.
References
-
- Bodelon G., Labrada L., Martinez-Costas J., Benavente J. The avian reovirus genome segment S1 is a functionally tricistronic gene that express one structural and two nonstructural proteins in infected cells. Virology. 2001;290:181–191. - PubMed
-
- Bodelon G., Labrada L., Martinez-Costas J., Benavente J. Modification of late membrane permeability in avian reovirus-infected cells: viroporin activity of the S1-encoded nonstructural p10 protein. J. Biol. Chem. 2002;277:17789–17796. - PubMed
-
- Chiu C.J., Lee L.J. Cloning and nucleotide sequencing of the S4 genome segment of avian reovirus S1133. Arch. Virol. 1997;142:2515–2520. - PubMed
-
- Chulu L.J., Lee L.H., Lee Y.C., Liao S.H., Shih W.L., Liu H.J. Apoptosis induction by avian reovirus through P53 and mitochondrial pathways. Biochem. Biophys. Res. Commun. 2007;356:529–535. - PubMed
-
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

