TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis
- PMID: 18455983
- PMCID: PMC2398615
- DOI: 10.1016/j.cell.2008.03.026
TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis
Abstract
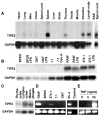
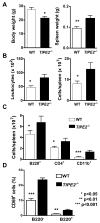
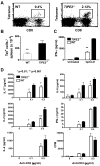
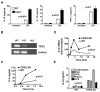
Immune homeostasis is essential for the normal functioning of the immune system, and its breakdown leads to fatal inflammatory diseases. We report here the identification of a member of the tumor necrosis factor-alpha-induced protein-8 (TNFAIP8) family, designated TIPE2, that is required for maintaining immune homeostasis. TIPE2 is preferentially expressed in lymphoid tissues, and its deletion in mice leads to multiorgan inflammation, splenomegaly, and premature death. TIPE2-deficient animals are hypersensitive to septic shock, and TIPE2-deficient cells are hyper-responsive to Toll-like receptor (TLR) and T cell receptor (TCR) activation. Importantly, TIPE2 binds to caspase-8 and inhibits activating protein-1 and nuclear factor-kappaB activation while promoting Fas-induced apoptosis. Inhibiting caspase-8 significantly blocks the hyper-responsiveness of TIPE2-deficient cells. These results establish that TIPE2 is an essential negative regulator of TLR and TCR function, and its selective expression in the immune system prevents hyperresponsiveness and maintains immune homeostasis.
Figures


Comment in
-
A different TIPE of immune homeostasis.Cell. 2008 May 2;133(3):401-2. doi: 10.1016/j.cell.2008.04.017. Cell. 2008. PMID: 18455981 Free PMC article.
References
-
- Bidere N, Snow AL, Sakai K, Zheng L, Lenardo MJ. Caspase-8 regulation by direct interaction with TRAF6 in T cell receptor-induced NF-kappaB activation. Current Biology. 2006;16:1666–1671. - PubMed
-
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annual Review of Cell & Developmental Biology. 1999;15:269–290. - PubMed
-
- Carmody R, Hilliard B, Chodosh L, Chen Y. Genomic scale profiling of autoimmune inflammation in the central nervous system: the nervous response to inflammation. Journal of Neuroimmunology. 2002;133:95–107. - PubMed
-
- Chaudhary PM, Eby MT, Jasmin A, Hood L. Activation of the c-Jun N-terminal kinase/stress-activated protein kinase pathway by overexpression of caspase-8 and its homologs. Journal of Biological Chemistry. 1999;274:19211–19219. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

