Virus growth and antibody responses following respiratory tract infection of ferrets and mice with WT and P/V mutants of the paramyxovirus Simian Virus 5
- PMID: 18456301
- PMCID: PMC2574746
- DOI: 10.1016/j.virol.2008.03.034
Virus growth and antibody responses following respiratory tract infection of ferrets and mice with WT and P/V mutants of the paramyxovirus Simian Virus 5
Abstract
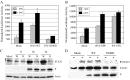
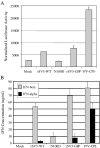
P/V gene substitutions convert the non-cytopathic paramyxovirus Simian Virus 5 (SV5), which is a poor inducer of host cell responses in human tissue culture cells, into a mutant (P/V-CPI-) that induces high levels of apoptosis, interferon (IFN)-beta, and proinflammatory cytokines. However, the effect of SV5-P/V gene mutations on virus growth and adaptive immune responses in animals has not been determined. Here, we used two distinct animal model systems to test the hypothesis that SV5-P/V mutants which are more potent activators of innate responses in tissue culture will also elicit higher antiviral antibody responses. In mouse cells, in vitro studies identified a panel of SV5-P/V mutants that ranged in their ability to limit IFN responses. Intranasal infection of mice with these WT and P/V mutant viruses elicited equivalent anti-SV5 IgG responses at all doses tested, and viral titers recovered from the respiratory tract were indistinguishable. In primary cultures of ferret lung fibroblasts, WT rSV5 and P/V-CPI- viruses had phenotypes similar to those established in human cell lines, including differential induction of IFN secretion, IFN signaling and apoptosis. Intranasal infection of ferrets with a low dose of WT rSV5 elicited approximately 500 fold higher anti-SV5 serum IgG responses compared to the P/V-CPI- mutant, and this correlated with overall higher viral titers for the WT virus in tracheal tissues. There was a dose-dependent increase in antibody response to infection of ferrets with P/V-CPI-, but not with WT rSV5. Together our data indicate that WT rSV5 and P/V mutants can elicit distinct innate and adaptive immunity phenotypes in the ferret animal model system, but not in the mouse system. We present a model for the effect of P/V gene substitutions on SV5 growth and immune responses in vivo.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

