Capsaicin-induced neuronal death and proliferation of the primary sensory neurons located in the nodose ganglia of adult rats
- PMID: 18456414
- PMCID: PMC2527584
- DOI: 10.1016/j.neuroscience.2008.03.055
Capsaicin-induced neuronal death and proliferation of the primary sensory neurons located in the nodose ganglia of adult rats
Abstract
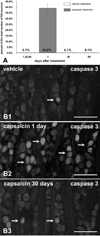
To evaluate the potential for neuronal replacement following destruction of vagal afferent neurons, we examined nodose ganglia following i.p. capsaicin treatment of adult rats. Rats received capsaicin or vehicle followed by a regimen of 5'-bromo-2'-deoxyuridine injections (BrdU) to reveal DNA replication. Nodose ganglia were harvested at various times post-treatment and processed for 4',6-diamidino-2-phenylindole (DAPI) nuclear staining and immunofluorescence to estimate neuronal numbers and to determine vanilloid receptor, cleaved caspase 3, TUNEL, BrdU, the neuron-selective marker protein gene product (PGP) -9.5 and neurofilament-M-immunoreactivity. Twenty-four hours after capsaicin approximately 40% of nodose ganglion neurons expressed cleaved caspase 3-immunoreactivity and 16% revealed TUNEL staining, indicating that primary sensory neurons are killed by the capsaicin treatment of adult rats. The occurrence of neuronal death was confirmed by counts of DAPI-stained neuronal nuclei, which revealed >or=50% reduction of nodose neuron number by 30 days post-capsaicin. However, by 60 days post-capsaicin, the total numbers of neuronal nuclei in nodose ganglia from capsaicin-treated rats were not different from controls, suggesting that new neurons had been added to the nodose ganglia. Neuronal proliferation was confirmed by significant BrdU incorporation in nuclei of nodose ganglion cells immunoreactive for the neuron-specific antigen PGP-9.5 revealed 30 and 60 days post-capsaicin. Collectively, these observations suggest that in adult rats massive scale neurogenesis occurs in nodose ganglia following capsaicin-induced neuronal destruction. The adult nodose ganglion, therefore, provides a novel system for studying neural plasticity and adult neurogenesis after peripheral injury of primary sensory neurons.
Figures




Similar articles
-
Plasticity of nodose ganglion neurons after capsaicin- and vagotomy-induced nerve damage in adult rats.Neuroscience. 2010 Jun 2;167(4):1227-38. doi: 10.1016/j.neuroscience.2010.02.049. Epub 2010 Mar 1. Neuroscience. 2010. PMID: 20197082
-
Neural proliferation and restoration of neurochemical phenotypes and compromised functions following capsaicin-induced neuronal damage in the nodose ganglion of the adult rat.Front Neurosci. 2011 Feb 2;5:12. doi: 10.3389/fnins.2011.00012. eCollection 2011. Front Neurosci. 2011. PMID: 21344007 Free PMC article.
-
Inhibition of axoplasmic transport in the rat vagus nerve alters the numbers of neuropeptide and tyrosine hydroxylase messenger RNA-containing and immunoreactive visceral afferent neurons of the nodose ganglion.Neuroscience. 1995 May;66(1):175-87. doi: 10.1016/0306-4522(94)00561-i. Neuroscience. 1995. PMID: 7543661
-
Neurotrophins alter the numbers of neurotransmitter-ir mature vagal/glossopharyngeal visceral afferent neurons in vitro.Brain Res. 2000 Nov 24;884(1--2):206-12. doi: 10.1016/s0006-8993(00)02988-7. Brain Res. 2000. PMID: 11082504
-
A quantitative investigation of the effects of neonatal capsaicin treatment on vagal afferent neurons in the rat.Cell Tissue Res. 1996 Feb;283(2):305-11. doi: 10.1007/s004410050540. Cell Tissue Res. 1996. PMID: 8593659
Cited by
-
Roux‑en‑Y gastric bypass surgery triggers rapid DNA fragmentation in vagal afferent neurons in rats.Acta Neurobiol Exp (Wars). 2019;79(4):432-444. Acta Neurobiol Exp (Wars). 2019. PMID: 31885399 Free PMC article.
-
Vagal afferent fibers contribute to the anti-inflammatory reactions by vagus nerve stimulation in concanavalin A model of hepatitis in rats.Mol Med. 2020 Dec 3;26(1):119. doi: 10.1186/s10020-020-00247-2. Mol Med. 2020. PMID: 33272194 Free PMC article.
-
Satellite glia of the adult dorsal root ganglia harbor stem cells that yield glia under physiological conditions and neurons in response to injury.Stem Cell Reports. 2022 Nov 8;17(11):2467-2483. doi: 10.1016/j.stemcr.2022.10.002. Stem Cell Reports. 2022. PMID: 36351367 Free PMC article.
-
Identification of Sodium Transients Through NaV1.5 Channels as Regulators of Differentiation in Immortalized Dorsal Root Ganglia Neurons.Front Cell Neurosci. 2022 Apr 6;16:816325. doi: 10.3389/fncel.2022.816325. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35465610 Free PMC article.
-
The controversial role of the vagus nerve in mediating ghrelin's actions: gut feelings and beyond.IBRO Neurosci Rep. 2022 Mar 12;12:228-239. doi: 10.1016/j.ibneur.2022.03.003. eCollection 2022 Jun. IBRO Neurosci Rep. 2022. PMID: 35746965 Free PMC article. Review.
References
-
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. - PubMed
-
- Bordey A. Adult neurogenesis: basic concepts of signaling. Cell Cycle. 2006;5:722–728. - PubMed
-
- Carobi C. A quantitative investigation of the effects of neonatal capsaicin treatment on vagal afferent neurons in the rat. Cell Tissue Res. 1996;283:305–311. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Chapouton P, Jagasia R, Bally-Cuif L. Adult neurogenesis in non-mammalian vertebrates. Bioessays. 2007;29:745–757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

