Structural and evolutionary classification of Type II restriction enzymes based on theoretical and experimental analyses
- PMID: 18456708
- PMCID: PMC2441816
- DOI: 10.1093/nar/gkn175
Structural and evolutionary classification of Type II restriction enzymes based on theoretical and experimental analyses
Abstract
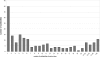
For a very long time, Type II restriction enzymes (REases) have been a paradigm of ORFans: proteins with no detectable similarity to each other and to any other protein in the database, despite common cellular and biochemical function. Crystallographic analyses published until January 2008 provided high-resolution structures for only 28 of 1637 Type II REase sequences available in the Restriction Enzyme database (REBASE). Among these structures, all but two possess catalytic domains with the common PD-(D/E)XK nuclease fold. Two structures are unrelated to the others: R.BfiI exhibits the phospholipase D (PLD) fold, while R.PabI has a new fold termed 'half-pipe'. Thus far, bioinformatic studies supported by site-directed mutagenesis have extended the number of tentatively assigned REase folds to five (now including also GIY-YIG and HNH folds identified earlier in homing endonucleases) and provided structural predictions for dozens of REase sequences without experimentally solved structures. Here, we present a comprehensive study of all Type II REase sequences available in REBASE together with their homologs detectable in the nonredundant and environmental samples databases at the NCBI. We present the summary and critical evaluation of structural assignments and predictions reported earlier, new classification of all REase sequences into families, domain architecture analysis and new predictions of three-dimensional folds. Among 289 experimentally characterized (not putative) Type II REases, whose apparently full-length sequences are available in REBASE, we assign 199 (69%) to contain the PD-(D/E)XK domain. The HNH domain is the second most common, with 24 (8%) members. When putative REases are taken into account, the fraction of PD-(D/E)XK and HNH folds changes to 48% and 30%, respectively. Fifty-six characterized (and 521 predicted) REases remain unassigned to any of the five REase folds identified so far, and may exhibit new architectures. These enzymes are proposed as the most interesting targets for structure determination by high-resolution experimental methods. Our analysis provides the first comprehensive map of sequence-structure relationships among Type II REases and will help to focus the efforts of structural and functional genomics of this large and biotechnologically important class of enzymes.
Figures


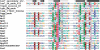
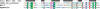
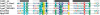


Similar articles
-
Type II restriction endonuclease R.Hpy188I belongs to the GIY-YIG nuclease superfamily, but exhibits an unusual active site.BMC Struct Biol. 2008 Nov 14;8:48. doi: 10.1186/1472-6807-8-48. BMC Struct Biol. 2008. PMID: 19014591 Free PMC article.
-
Type II restriction endonuclease R.Eco29kI is a member of the GIY-YIG nuclease superfamily.BMC Struct Biol. 2007 Jul 12;7:48. doi: 10.1186/1472-6807-7-48. BMC Struct Biol. 2007. PMID: 17626614 Free PMC article.
-
Theoretical model of restriction endonuclease HpaI in complex with DNA, predicted by fold recognition and validated by site-directed mutagenesis.Proteins. 2006 Jun 1;63(4):1059-68. doi: 10.1002/prot.20920. Proteins. 2006. PMID: 16498623
-
Crystallographic and bioinformatic studies on restriction endonucleases: inference of evolutionary relationships in the "midnight zone" of homology.Curr Protein Pept Sci. 2003 Oct;4(5):327-37. doi: 10.2174/1389203033487072. Curr Protein Pept Sci. 2003. PMID: 14529527 Review.
-
Categoric prediction of metal ion mechanisms in the active sites of 17 select type II restriction endonucleases.Biochem Biophys Res Commun. 2010 Nov 12;402(2):177-9. doi: 10.1016/j.bbrc.2010.09.113. Epub 2010 Oct 1. Biochem Biophys Res Commun. 2010. PMID: 20888795 Review.
Cited by
-
Hpy188I-DNA pre- and post-cleavage complexes--snapshots of the GIY-YIG nuclease mediated catalysis.Nucleic Acids Res. 2011 Mar;39(4):1554-64. doi: 10.1093/nar/gkq821. Epub 2010 Oct 8. Nucleic Acids Res. 2011. PMID: 20935048 Free PMC article.
-
Functional analysis of MmeI from methanol utilizer Methylophilus methylotrophus, a subtype IIC restriction-modification enzyme related to type I enzymes.Appl Environ Microbiol. 2009 Jan;75(1):212-23. doi: 10.1128/AEM.01322-08. Epub 2008 Nov 7. Appl Environ Microbiol. 2009. PMID: 18997032 Free PMC article.
-
Crystal structure and DNA cleavage mechanism of the restriction DNA glycosylase R.CcoLI from Campylobacter coli.Sci Rep. 2021 Jan 13;11(1):859. doi: 10.1038/s41598-020-79537-y. Sci Rep. 2021. PMID: 33441677 Free PMC article.
-
Live virus-free or die: coupling of antivirus immunity and programmed suicide or dormancy in prokaryotes.Biol Direct. 2012 Nov 14;7:40. doi: 10.1186/1745-6150-7-40. Biol Direct. 2012. PMID: 23151069 Free PMC article.
-
Type I restriction enzymes and their relatives.Nucleic Acids Res. 2014 Jan;42(1):20-44. doi: 10.1093/nar/gkt847. Epub 2013 Sep 24. Nucleic Acids Res. 2014. PMID: 24068554 Free PMC article. Review.
References
-
- Skowronek KJ, Bujnicki JM. In: Industrial Enzymes: Structure, Function and Applications. Polaina J, MacCabe AP, editors. Springer; 2007. Chapter 21.
-
- Williams RJ. Restriction endonucleases: classification, properties, and applications. Mol. Biotechnol. 2003;23:225–243. - PubMed
-
- Pingoud AM. Restriction Endonucleases. Berlin, Heidelberg: Springer; 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

