Cardiac phosphatase-deficient 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase increases glycolysis, hypertrophy, and myocyte resistance to hypoxia
- PMID: 18456722
- PMCID: PMC4239994
- DOI: 10.1152/ajpheart.91501.2007
Cardiac phosphatase-deficient 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase increases glycolysis, hypertrophy, and myocyte resistance to hypoxia
Abstract
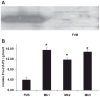
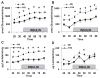
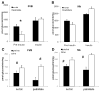
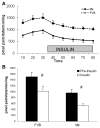
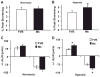
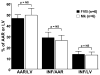
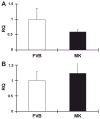
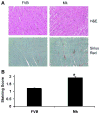
During ischemia and heart failure, there is an increase in cardiac glycolysis. To understand if this is beneficial or detrimental to the heart, we chronically elevated glycolysis by cardiac-specific overexpression of phosphatase-deficient 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2) in transgenic mice. PFK-2 controls the level of fructose-2,6-bisphosphate (Fru-2,6-P2), an important regulator of phosphofructokinase and glycolysis. Transgenic mice had over a threefold elevation in levels of Fru-2,6-P2. Cardiac metabolites upstream of phosphofructokinase were significantly reduced, as would be expected by the activation of phosphofructokinase. In perfused hearts, the transgene caused a significant increase in glycolysis that was less sensitive to inhibition by palmitate. Conversely, oxidation of palmitate was reduced by close to 50%. The elevation in glycolysis made isolated cardiomyocytes highly resistant to contractile inhibition by hypoxia, but in vivo the transgene had no effect on ischemia-reperfusion injury. Transgenic hearts exhibited pathology: the heart weight-to-body weight ratio was increased 17%, cardiomyocyte length was greater, and cardiac fibrosis was increased. However, the transgene did not change insulin sensitivity. These results show that the elevation in glycolysis provides acute benefits against hypoxia, but the chronic increase in glycolysis or reduction in fatty acid oxidation interferes with normal cardiac metabolism, which may be detrimental to the heart.
Figures
References
-
- Augustus AS, Buchanan J, Park TS, Hirata K, Noh HL, Sun J, Homma S, D’Armiento J, Abel ED, Goldberg IJ. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem. 2006;281:8716–8723. - PubMed
-
- Beauloye C, Marsin AS, Bertrand L, Vanoverschelde JL, Rider MH, Hue L. The stimulation of heart glycolysis by increased workload does not require AMP-activated protein kinase but a wortmannin-sensitive mechanism. FEBS Lett. 2002;531:324–328. - PubMed
-
- Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. - PubMed
-
- Belke DD, Larsen TS, Gibbs EM, Severson DL. Glucose metabolism in perfused mouse hearts overexpressing human GLUT-4 glucose transporter. Am J Physiol Endocrinol Metab. 2001;280:E420–E427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

