Subepicardial phase 0 block and discontinuous transmural conduction underlie right precordial ST-segment elevation by a SCN5A loss-of-function mutation
- PMID: 18456723
- PMCID: PMC2494753
- DOI: 10.1152/ajpheart.91495.2007
Subepicardial phase 0 block and discontinuous transmural conduction underlie right precordial ST-segment elevation by a SCN5A loss-of-function mutation
Abstract
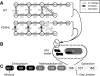
Two mechanisms are generally proposed to explain right precordial ST-segment elevation in Brugada syndrome: 1) right ventricular (RV) subepicardial action potential shortening and/or loss of dome causing transmural dispersion of repolarization; and 2) RV conduction delay. Here we report novel mechanistic insights into ST-segment elevation associated with a Na(+) current (I(Na)) loss-of-function mutation from studies in a Dutch kindred with the COOH-terminal SCN5A variant p.Phe2004Leu. The proband, a man, experienced syncope at age 22 yr and had coved-type ST-segment elevations in ECG leads V1 and V2 and negative T waves in V2. Peak and persistent mutant I(Na) were significantly decreased. I(Na) closed-state inactivation was increased, slow inactivation accelerated, and recovery from inactivation delayed. Computer-simulated I(Na)-dependent excitation was decremental from endo- to epicardium at cycle length 1,000 ms, not at cycle length 300 ms. Propagation was discontinuous across the midmyocardial to epicardial transition region, exhibiting a long local delay due to phase 0 block. Beyond this region, axial excitatory current was provided by phase 2 (dome) of the M-cell action potentials and depended on L-type Ca(2+) current ("phase 2 conduction"). These results explain right precordial ST-segment elevation on the basis of RV transmural gradients of membrane potentials during early repolarization caused by discontinuous conduction. The late slow-upstroke action potentials at the subepicardium produce T-wave inversion in the computed ECG waveform, in line with the clinical ECG.
Figures


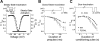
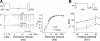

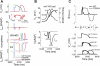
Comment in
-
The complexity of genotype-phenotype relations associated with loss-of-function sodium channel mutations and the role of in silico studies.Am J Physiol Heart Circ Physiol. 2008 Jul;295(1):H8-9. doi: 10.1152/ajpheart.00494.2008. Epub 2008 May 23. Am J Physiol Heart Circ Physiol. 2008. PMID: 18502906 No abstract available.
References
-
- Ackerman MJ, Splawski I, Makielski JC, Tester DJ, Will ML, Timothy KW, Keating MT, Jones G, Chadha M, Burrow CR, Stephens JC, Xu C, Judson R, Curran ME. Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian, and Hispanic individuals: implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm 1: 600–607, 2004. - PubMed
-
- Albert CM, Nam EG, Rimm EB, Jin HW, Hajjar RJ, Hunter DJ, MacRae CA, Ellinor PT. Cardiac sodium channel gene variants and sudden cardiac death in women. Circulation 117: 16–23, 2008. - PubMed
-
- Antzelevitch C Late potentials and the Brugada syndrome. J Am Coll Cardiol 39: 1996–1999, 2002. - PubMed
-
- Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A. Brugada syndrome: report of the second consensus conference. Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 111: 659–670, 2005. - PubMed
-
- Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu DW. Heterogeneity within the ventricular wall: electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res 69: 1427–1449, 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

