Tone-dependent vascular responses to astrocyte-derived signals
- PMID: 18456724
- PMCID: PMC3962545
- DOI: 10.1152/ajpheart.91451.2007
Tone-dependent vascular responses to astrocyte-derived signals
Abstract
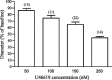
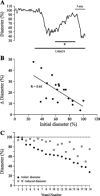
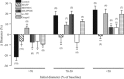
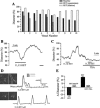
A growing number of studies support an important contribution of astrocytes to neurovascular coupling, i.e., the phenomenon by which variations in neuronal activity trigger localized changes in blood flow that serve to match the metabolic demands of neurons. However, since both constriction and dilations have been observed in brain parenchymal arterioles upon astrocyte stimulation, the specific influences of these cells on the vasculature remain unclear. Using acute brain slices, we present evidence showing that the specific degree of constriction of rat cortical arterioles (vascular tone) is a key determinant of the magnitude and polarity of the diameter changes elicited by signals associated with neurovascular coupling. Thus elevation of extracellular K+ concentration, stimulation of metabotropic glutamate receptors (mGluR), or 11,12-epoxyeicosatrienoic acid application all elicited vascular responses that were affected by the particular resting arteriolar tone. Interestingly, the data suggest that the extent and/or polarity of the vascular responses are influenced by a delimited set point centered between 30 and 40% tone. In addition, we report that distinct, tone-dependent effects on arteriolar diameter occur upon stimulation of mGluR during inhibition of enzymes of the arachidonic acid pathway [i.e., phospholipase A2, cytochrome P-450 (CYP) omega-hydroxylase, CYP epoxygenase, and cycloxygenase-1]. Our findings may reconcile previous evidence in which direct astrocytic stimulation elicited either vasoconstrictions or vasodilations and also suggest the novel concept that, in addition to participating in functional hyperemia, astrocyte-derived signals play a role in adjusting vascular tone to a range where dilator responses are optimal.
Figures

References
-
- AlkayedNJAlkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke 28: 1066–1072, 1997. - PubMed
-
- AlkayedNJAlkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke 27: 971–979, 1996. - PubMed
-
- ArmsteadWMArmstead WM, Mirro R, Busija DW, Leffler CW. Vascular responses to vasopressin are tone-dependent in the cerebral circulation of the newborn pig. Circ Res 64: 136–144, 1989. - PubMed
-
- BarmanSABarman SA, Zhu S, Han G, White RE. cAMP activates BKCa channels in pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 284: L1004–L1011, 2003. - PubMed
-
- BhardwajABhardwaj A, Northington FJ, Carhuapoma JR, Falck JR, Harder DR, Traystman RJ, Koehler RC. P-450 epoxygenase and NO synthase inhibitors reduce cerebral blood flow response to N-methyl-d-aspartate. Am J Physiol Heart Circ Physiol 279: H1616–H1624, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

