Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats
- PMID: 18456730
- PMCID: PMC2494740
- DOI: 10.1152/ajpheart.01277.2007
Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats
Abstract
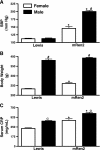
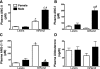
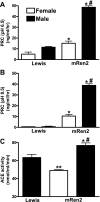
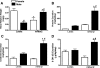
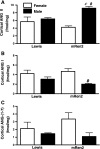
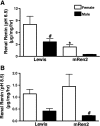
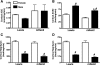
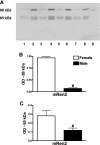
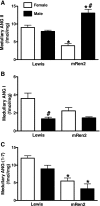
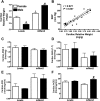
Sex differences in blood pressure are evident in experimental models and human subjects, yet the mechanisms underlying this disparity remain equivocal. The current study sought to define the extent of male-female differences in the circulating and tissue renin-angiotensin aldosterone systems (RAASs) of congenic mRen(2). Lewis and control Lewis rats. Male congenics exhibited higher systolic blood pressure than females [200 +/- 4 vs. 146 +/- 7 mmHg, P < 0.01] or Lewis males and females [113 +/- 2 vs. 112 +/- 2 mmHg, P > 0.05]. Plasma ANG II levels were twofold higher in male congenics [47 +/- 3 vs. 19 +/- 3 pM, P < 0.01] and fivefold higher than in male or female Lewis rats [6 +/- 1 vs. 6 +/- 1 pM]. ANG I levels were also highest in the males; however, plasma ANG-(1-7) was higher in female congenics. Male congenics exhibited greater circulating renin and angiotensin-converting enzyme (ACE) activities, as well as angiotensinogen, than female littermates. Renal cortical and medullary ANG II levels were also higher in the male congenics versus all the other groups; ANG I was lower in the males. Cortical ACE2 activity was higher in male congenics, yet neprilysin activity and protein were greater in the females, which may contribute to reduced renal levels of ANG II. These data reveal that sex differences in both the circulating and renal RAAS are apparent primarily in the hypertensive group. The enhanced activity of the RAAS in male congenics may contribute to the higher pressure and tissue injury evident in the strain.
Figures

References
-
- Allred AJ, Chappell MC, Ferrario CM, Diz DI. Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol 279: F636–F645, 2000. - PubMed
-
- Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1-7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol 279: F841–F850, 2000. - PubMed
-
- Armando I, Jezova M, Juorio AV, Terron JA, Falcon-Neri A, Semino-Mora C, Imboden H, Saavedra JM. Estrogen upregulates renal angiotensin II AT2 receptors. Am J Physiol Renal Physiol 283: F934–F943, 2002. - PubMed
-
- August P, Oparil S. Hypertension in women. J Clin Endocrinol Metab 84: 1862–1866, 1999. - PubMed
-
- Bachmann J, Feldmer M, Ganten U, Stock G, Ganten D. Sexual dimorphism of blood pressure: possible role of the renin-angiotensin system. J Steroid Biochem 40: 511–515, 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

