Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, alphavbeta3-integrin, and TGF-beta1 in response to ANG II and high glucose
- PMID: 18456735
- PMCID: PMC2494762
- DOI: 10.1152/ajpheart.00341.2008
Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, alphavbeta3-integrin, and TGF-beta1 in response to ANG II and high glucose
Abstract
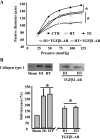
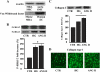
This study determines that vascular smooth muscle cell (VSMC) signaling through extracellular signal-regulated kinase (ERK) 1/2-mitogen-activated protein (MAP) kinase, alphavbeta(3)-integrin, and transforming growth factor (TGF)-beta1 dictates collagen type I network induction in mesenteric resistance arteries (MRA) from type 1 diabetic (streptozotocin) or hypertensive (HT; ANG II) mice. Isolated MRA were subjected to a pressure-passive-diameter relationship. To delineate cell types and mechanisms, cultured VSMC were prepared from MRA and stimulated with ANG II (100 nM) and high glucose (HG, 22 mM). Pressure-passive-diameter relationship reduction was associated with increased collagen type I deposition in MRA from HT and diabetic mice compared with control. Treatment of HT and diabetic mice with neutralizing TGF-beta1 antibody reduced MRA stiffness and collagen type I deposition. Cultured VSMC stimulated with HG or ANG II for 5 min increased ERK1/2-MAP kinase phosphorylation, whereas a 48-h stimulation induced latent TGF-beta1, alphavbeta(3)-integrin, and collagen type 1 release in the conditioned media. TGF-beta1 bioactivity and Smad2 phosphorylation were alphavbeta(3)-integrin-dependent, since beta(3)-integrin antibody and alphavbeta(3)-integrin inhibitor (SB-223245, 10 microM) significantly prevented TGF-beta1 bioactivity and Smad2 phosphorylation. Pretreatment of VSMC with ERK1/2-MAP kinase inhibitor (U-0126, 1 microM) reduced alphavbeta(3)-integrin, TGF-beta1, and collagen type 1 content. Additionally, alphavbeta(3)-integrin antibody, SB-223245, TGF-beta1-small-intefering RNA (siRNA), and Smad2-siRNA (40 nM) prevented collagen type I network formation in response to ANG II and HG. Together, these data provide evidence that resistance artery fibrosis in type 1 diabetes and hypertension is a consequence of abnormal collagen type I release by VSMC and involves ERK1/2, alphavbeta(3)-integrin, and TGF-beta1 signaling. This pathway could be a potential target for overcoming small artery complications in diabetes and hypertension.
Figures
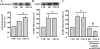



Similar articles
-
Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3.Hypertension. 2009 Oct;54(4):877-84. doi: 10.1161/HYPERTENSIONAHA.109.136531. Epub 2009 Aug 10. Hypertension. 2009. PMID: 19667256
-
p38 Map kinase regulates vascular smooth muscle cell collagen synthesis by angiotensin II in SHR but not in WKY.Hypertension. 2001 Feb;37(2 Pt 2):574-80. doi: 10.1161/01.hyp.37.2.574. Hypertension. 2001. PMID: 11230337
-
TGF-beta inhibits Ang II-induced MAPK p44/42 signaling in vascular smooth muscle cells by Ang II type 1 receptor downregulation.J Vasc Res. 2009;46(5):459-68. doi: 10.1159/000200961. Epub 2009 Feb 10. J Vasc Res. 2009. PMID: 19204403
-
Integration of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells.Thromb Haemost. 2008 Dec;100(6):976-83. Thromb Haemost. 2008. PMID: 19132220 Free PMC article. Review.
-
Angiotensin II, Hypercholesterolemia, and Vascular Smooth Muscle Cells: A Perfect Trio for Vascular Pathology.Int J Mol Sci. 2020 Jun 25;21(12):4525. doi: 10.3390/ijms21124525. Int J Mol Sci. 2020. PMID: 32630530 Free PMC article. Review.
Cited by
-
Thrombin-mediated proteoglycan synthesis utilizes both protein-tyrosine kinase and serine/threonine kinase receptor transactivation in vascular smooth muscle cells.J Biol Chem. 2013 Mar 8;288(10):7410-9. doi: 10.1074/jbc.M112.400259. Epub 2013 Jan 18. J Biol Chem. 2013. PMID: 23335513 Free PMC article.
-
It is all in the blood: the multifaceted contribution of circulating progenitor cells in diabetic complications.Exp Diabetes Res. 2012;2012:742976. doi: 10.1155/2012/742976. Epub 2012 Apr 3. Exp Diabetes Res. 2012. PMID: 22548049 Free PMC article. Review.
-
Poly(ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation.J Immunol. 2010 Aug 1;185(3):1894-902. doi: 10.4049/jimmunol.1000646. Epub 2010 Jul 7. J Immunol. 2010. PMID: 20610652 Free PMC article.
-
Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice.Lab Invest. 2010 Jul;90(7):985-96. doi: 10.1038/labinvest.2010.86. Epub 2010 May 3. Lab Invest. 2010. PMID: 20440273 Free PMC article.
-
Vascular wall extracellular matrix proteins and vascular diseases.Biochim Biophys Acta. 2014 Nov;1842(11):2106-2119. doi: 10.1016/j.bbadis.2014.07.008. Epub 2014 Jul 18. Biochim Biophys Acta. 2014. PMID: 25045854 Free PMC article. Review.
References
-
- Bernadet-Monrozies P, Rostaing L, Kamar N, Durand D. The effect of angiotensin-converting enzyme inhibitors on the progression of chronic renal failure. Presse Med 31: 1714–1720, 2002. - PubMed
-
- Breindl M, Harbers K, Jaenisch R. Retrovirus-induced lethal mutation in collagen I gene of mice is associated with an altered chromatin structure. Cell 38: 9–16, 1984. - PubMed
-
- Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes 54: 1829–1837, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

