Mutagenesis of the Shigella flexneri autotransporter IcsA reveals novel functional regions involved in IcsA biogenesis and recruitment of host neural Wiscott-Aldrich syndrome protein
- PMID: 18456802
- PMCID: PMC2446779
- DOI: 10.1128/JB.00093-08
Mutagenesis of the Shigella flexneri autotransporter IcsA reveals novel functional regions involved in IcsA biogenesis and recruitment of host neural Wiscott-Aldrich syndrome protein
Abstract
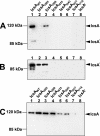
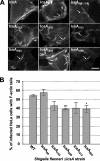
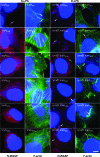
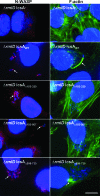
The IcsA (VirG) protein of Shigella flexneri is a polarly localized, outer membrane protein that is essential for virulence. Within host cells, IcsA activates the host actin regulatory protein, neural Wiskott-Aldrich syndrome protein (N-WASP), which in turn recruits the Arp2/3 complex, which nucleates host actin to form F-actin comet tails and initiate bacterial motility. Linker insertion mutagenesis was undertaken to randomly introduce 5-amino-acid in-frame insertions within IcsA. Forty-seven linker insertion mutants were isolated and expressed in S. flexneri Delta icsA strains. Mutants were characterized for IcsA protein production, cell surface expression and localization, intercellular spreading, F-actin comet tail formation, and N-WASP recruitment. Using this approach, we have identified a putative autochaperone region required for IcsA biogenesis, and our data suggest an additional region, not previously identified, is required for N-WASP recruitment.
Figures



References
-
- Baker, S., J. Gunn, and R. Morona. 1999. The Salmonella typhi melittin resistance gene pqaB affects intracellular growth in PMA-differentiated U937 cells, polymyxin B resistance and lipopolysaccharide. Microbiology 145367-378. - PubMed
-
- Berthiaume, F., N. Rutherford, and M. Mourez. 2007. Mutations affecting the biogenesis of the AIDA-I autotransporter. Res. Microbiol. 158348-354. - PubMed
-
- Brahmbhatt, H. N., A. A. Lindberg, and K. N. Timmis. 1992. Shigella lipopolysaccharide: structure, genetics, and vaccine development. Curr. Top. Microbiol. Immunol. 18045-64. - PubMed
-
- Brandon, L. D., N. Goehring, A. Janakiraman, A. W. Yan, T. Wu, J. Beckwith, and M. B. Goldberg. 2003. IcsA, a polarly localized autotransporter with an atypical signal peptide, uses the Sec apparatus for secretion, although the Sec apparatus is circumferentially distributed. Mol. Microbiol. 5045-60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

