Temperature sensitivity and cell division defects in an Escherichia coli strain with mutations in yghB and yqjA, encoding related and conserved inner membrane proteins
- PMID: 18456815
- PMCID: PMC2446817
- DOI: 10.1128/JB.00414-08
Temperature sensitivity and cell division defects in an Escherichia coli strain with mutations in yghB and yqjA, encoding related and conserved inner membrane proteins
Abstract
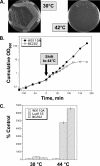
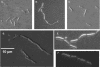
Ludox density gradients were used to enrich for Escherichia coli mutants with conditional growth defects and alterations in membrane composition. A temperature-sensitive mutant named Lud135 was isolated with mutations in two related, nonessential genes: yghB and yqjA. yghB harbors a single missense mutation (G203D) and yqjA contains a nonsense mutation (W92TGA) in Lud135. Both mutations are required for the temperature-sensitive phenotype: targeted deletion of both genes in a wild-type background results in a strain with a similar phenotype and expression of either gene from a plasmid restores growth at elevated temperatures. The mutant has altered membrane phospholipid levels, with elevated levels of acidic phospholipids, when grown under permissive conditions. Growth of Lud135 under nonpermissive conditions is restored by the presence of millimolar concentrations of divalent cations Ca(2+), Ba(2+), Sr(2+), or Mg(2+) or 300 to 500 mM NaCl but not 400 mM sucrose. Microscopic analysis of Lud135 demonstrates a dramatic defect at a late stage of cell division when cells are grown under permissive conditions. yghB and yqjA belong to the conserved and widely distributed dedA gene family, for which no function has been reported. The two open reading frames encode predicted polytopic inner membrane proteins with 61% amino acid identity. It is likely that YghB and YqjA play redundant but critical roles in membrane biology that are essential for completion of cell division in E. coli.
Figures





Similar articles
-
Escherichia coli YqjA, a Member of the Conserved DedA/Tvp38 Membrane Protein Family, Is a Putative Osmosensing Transporter Required for Growth at Alkaline pH.J Bacteriol. 2015 Jul;197(14):2292-300. doi: 10.1128/JB.00175-15. Epub 2015 Apr 27. J Bacteriol. 2015. PMID: 25917916 Free PMC article.
-
Klebsiella pneumoniae DedA family proteins have redundant roles in divalent cation homeostasis and resistance to phagocytosis.Microbiol Spectr. 2024 Feb 6;12(2):e0380723. doi: 10.1128/spectrum.03807-23. Epub 2024 Jan 12. Microbiol Spectr. 2024. PMID: 38214522 Free PMC article.
-
Inefficient Tat-dependent export of periplasmic amidases in an Escherichia coli strain with mutations in two DedA family genes.J Bacteriol. 2010 Feb;192(3):807-18. doi: 10.1128/JB.00716-09. Epub 2009 Oct 30. J Bacteriol. 2010. PMID: 19880597 Free PMC article.
-
New functions for the ancient DedA membrane protein family.J Bacteriol. 2013 Jan;195(1):3-11. doi: 10.1128/JB.01006-12. Epub 2012 Oct 19. J Bacteriol. 2013. PMID: 23086209 Free PMC article. Review.
-
Escherichia coli Small Proteome.EcoSal Plus. 2020 May;9(1):10.1128/ecosalplus.ESP-0031-2019. doi: 10.1128/ecosalplus.ESP-0031-2019. EcoSal Plus. 2020. PMID: 32385980 Free PMC article. Review.
Cited by
-
A DedA Family Membrane Protein in Indium Extrusion in Rhodanobacter sp. B2A1Ga4.Front Microbiol. 2021 Nov 26;12:772127. doi: 10.3389/fmicb.2021.772127. eCollection 2021. Front Microbiol. 2021. PMID: 34925279 Free PMC article.
-
Evolutionary engineering of E. coli MG1655 for tolerance against isoprenol.Biotechnol Biofuels. 2020 Nov 9;13(1):183. doi: 10.1186/s13068-020-01825-6. Biotechnol Biofuels. 2020. PMID: 33292484 Free PMC article.
-
A link between pH homeostasis and colistin resistance in bacteria.Sci Rep. 2021 Jun 24;11(1):13230. doi: 10.1038/s41598-021-92718-7. Sci Rep. 2021. PMID: 34168215 Free PMC article.
-
MzrA: a novel modulator of the EnvZ/OmpR two-component regulon.Mol Microbiol. 2009 Jun;72(6):1408-22. doi: 10.1111/j.1365-2958.2009.06728.x. Epub 2009 May 8. Mol Microbiol. 2009. PMID: 19432797 Free PMC article.
-
Cyanobacterial membrane dynamics in the light of eukaryotic principles.Biosci Rep. 2023 Feb 27;43(2):BSR20221269. doi: 10.1042/BSR20221269. Biosci Rep. 2023. PMID: 36602300 Free PMC article.
References
-
- Barr, F. A., and U. Gruneberg. 2007. Cytokinesis: placing and making the final cut. Cell 131847-860. - PubMed
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 2771453-1474. - PubMed
-
- Butland, G., J. M. Peregrin-Alvarez, J. Li, W. Yang, X. Yang, V. Canadien, A. Starostine, D. Richards, B. Beattie, N. Krogan, M. Davey, J. Parkinson, J. Greenblatt, and A. Emili. 2005. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 433531-537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

