Biophysical characterization of the unstructured cytoplasmic domain of the human neuronal adhesion protein neuroligin 3
- PMID: 18456828
- PMCID: PMC2483779
- DOI: 10.1529/biophysj.107.126995
Biophysical characterization of the unstructured cytoplasmic domain of the human neuronal adhesion protein neuroligin 3
Abstract
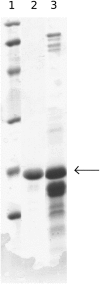
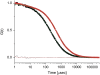
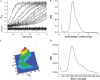
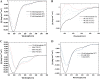
Cholinesterase-like adhesion molecules (CLAMs) are a family of neuronal cell adhesion molecules with important roles in synaptogenesis, and in maintaining structural and functional integrity of the nervous system. Our earlier study on the cytoplasmic domain of one of these CLAMs, the Drosophila protein, gliotactin, showed that it is intrinsically unstructured in vitro. Bioinformatic analysis suggested that the cytoplasmic domains of other CLAMs are also intrinsically unstructured, even though they bear no sequence homology to each other or to any known protein. In this study, we overexpress and purify the cytoplasmic domain of human neuroligin 3, notwithstanding its high sensitivity to the Escherichia coli endogenous proteases that cause its rapid degradation. Using bioinformatic analysis, sensitivity to proteases, size exclusion chromatography, fluorescence correlation spectroscopy, analytical ultracentrifugation, small angle x-ray scattering, circular dichroism, electron spin resonance, and nuclear magnetic resonance, we show that the cytoplasmic domain of human neuroligin 3 is intrinsically unstructured. However, several of these techniques indicate that it is not fully extended, but becomes significantly more extended under denaturing conditions.
Figures





References
-
- Botti, S. A., C. E. Felder, J. L. Sussman, and I. Silman. 1998. Electrotactins: a class of adhesion proteins with conserved electrostatic and structural motifs. Protein Eng. 11:415–420. - PubMed
-
- Gilbert, M. M., and V. J. Auld. 2005. Evolution of clams (cholinesterase-like adhesion molecules): structure and function during development. Front. Biosci. 10:2177–2192. - PubMed
-
- Ichtchenko, K., Y. Hata, T. Nguyen, B. Ullrich, M. Missler, C. Moomaw, and T. C. Sudhof. 1995. Neuroligin 1: a splice site-specific ligand for β-neurexins. Cell. 81:435–443. - PubMed
-
- Gilbert, M., J. Smith, A. J. Roskams, and V. J. Auld. 2001. Neuroligin 3 is a vertebrate gliotactin expressed in the olfactory ensheathing glia, a growth-promoting class of macroglia. Glia. 34:151–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

