Competition between the Rex1 exonuclease and the La protein affects both Trf4p-mediated RNA quality control and pre-tRNA maturation
- PMID: 18456844
- PMCID: PMC2390804
- DOI: 10.1261/rna.1050408
Competition between the Rex1 exonuclease and the La protein affects both Trf4p-mediated RNA quality control and pre-tRNA maturation
Abstract
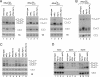
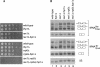
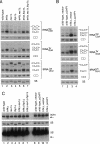
Although nascent noncoding RNAs can undergo maturation to functional RNAs or degradation by quality control pathways, the events that influence the choice of pathway are not understood. We report that the targeting of pre-tRNAs and certain other noncoding RNAs for decay by the TRAMP pathway is strongly influenced by competition between the La protein and the Rex1 exonuclease for access to their 3' ends. The La protein binds the 3' ends of many nascent noncoding RNAs, protecting them from exonucleases. We demonstrate that unspliced, end-matured, partially aminoacylated pre-tRNAs accumulate in yeast lacking the TRAMP subunit Trf4p, indicating that these pre-tRNAs normally undergo decay. By comparing RNA extracted from wild-type and mutant yeast strains, we show that Rex1p is the major exonuclease involved in pre-tRNA trailer trimming and may also function in nuclear CCA turnover. As the accumulation of end-matured pre-tRNAs in trf4Delta cells requires Rex1p, these pre-tRNAs are formed by exonucleolytic trimming. Accumulation of truncated forms of 5S rRNA and SRP RNA in trf4Delta cells also requires Rex1p. Overexpression of the La protein Lhp1p reduces both exonucleolytic pre-tRNA trimming in wild-type cells and the accumulation of defective RNAs in trf4Delta cells. Our experiments reveal that one consequence of Rex1p-dependent 3' trimming is the generation of aberrant RNAs that are targeted for decay by TRAMP.
Figures
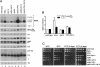


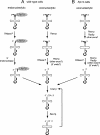
References
-
- Aebi M., Kirchner G., Chen J.Y., Vijayraghavan U., Jacobson A., Martin N.C., Abelson J. Isolation of a temperature-sensitive mutant with an altered tRNA nucleotidyltransferase and cloning of the gene encoding tRNA nucleotidyltransferase in the yeast Saccharomyces cerevisiae . J. Biol. Chem. 1990;25:16216–16220. - PubMed
-
- Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K. Current protocols in molecular biology. John Wiley & Sons; New York: 1998.
-
- Briggs M.W., Burkard K.T., Butler J.S. Rrp6p, the yeast homologue of the human PM-Scl 100-kDa autoantigen, is essential for efficient 5.8 S rRNA 3′ end formation. J. Biol. Chem. 1998;273:13255–13263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
