The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice
- PMID: 18457598
- PMCID: PMC2396659
- DOI: 10.1186/1755-8794-1-14
The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice
Abstract
Background: Obesity and insulin resistance are two major risk factors underlying the metabolic syndrome. The development of these metabolic disorders is frequently studied, but mainly in liver, skeletal muscle, and adipose tissue. To gain more insight in the role of the small intestine in development of obesity and insulin resistance, dietary fat-induced differential gene expression was determined along the longitudinal axis of small intestines of C57BL/6J mice.
Methods: Male C57BL/6J mice were fed a low-fat or a high-fat diet that mimicked the fatty acid composition of a Western-style human diet. After 2, 4 and 8 weeks of diet intervention small intestines were isolated and divided in three equal parts. Differential gene expression was determined in mucosal scrapings using Mouse genome 430 2.0 arrays.
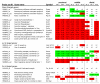
Results: The high-fat diet significantly increased body weight and decreased oral glucose tolerance, indicating insulin resistance. Microarray analysis showed that dietary fat had the most pronounced effect on differential gene expression in the middle part of the small intestine. By overrepresentation analysis we found that the most modulated biological processes on a high-fat diet were related to lipid metabolism, cell cycle and inflammation. Our results further indicated that the nuclear receptors Ppars, Lxrs and Fxr play an important regulatory role in the response of the small intestine to the high-fat diet. Next to these more local dietary fat effects, a secretome analysis revealed differential gene expression of secreted proteins, such as Il18, Fgf15, Mif, Igfbp3 and Angptl4. Finally, we linked the fat-induced molecular changes in the small intestine to development of obesity and insulin resistance.
Conclusion: During dietary fat-induced development of obesity and insulin resistance, we found substantial changes in gene expression in the small intestine, indicating modulations of biological processes, especially related to lipid metabolism. Moreover, we found differential expression of potential signaling molecules that can provoke systemic effects in peripheral organs by influencing their metabolic homeostasis. Many of these fat-modulated genes could be linked to obesity and/or insulin resistance. Together, our data provided various leads for a causal role of the small intestine in the etiology of obesity and/or insulin resistance.
Figures





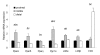

References
-
- Kondo H, Minegishi Y, Komine Y, Mori T, Matsumoto I, Abe K, Tokimitsu I, Hase T, Murase T. Differential regulation of intestinal lipid metabolism-related genes in obesity-resistant A/J vs. obesity-prone C57BL/6J mice. Am J Physiol Endocrinol Metab. 2006;291:E1092–1099. doi: 10.1152/ajpendo.00583.2005. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

