Identification of a consensus site for TRAF6/p62 polyubiquitination
- PMID: 18457658
- PMCID: PMC2474794
- DOI: 10.1016/j.bbrc.2008.04.138
Identification of a consensus site for TRAF6/p62 polyubiquitination
Abstract
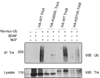
Tumor necrosis factor receptor-associated factor 6 (TRAF6) is an ubiquitin ligase that regulates a diverse array of physiological processes via forming Lys-63 linked polyubiquitin chains. In this study, the lysine selection process for TRAF6/p62 ubiquitination was examined. The protein sequence of two characterized TRAF6/p62 substrates, NRIF and TrkA, revealed a conserved consensus pattern for the ubiquitination site of these two TRAF6 substrates. The consensus pattern established in the verified substrates was common to the other Trk receptor family members, TrkB and TrkC. Interestingly, Lysine 811 in TrkB was selected for ubiquination, and mutation of Lysine 811 diminished the formation of TRAF6/p62 complex that is necessary for effective ubiquination. Moreover, downstream signaling was affected upon binding of BDNF to the mutant TrkB receptor. These findings reveal a possible selection process for targeting a specific lysine residue by a single E3 ligase and underscore the role of the scaffold, p62, in this process.
Figures



Similar articles
-
Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling.Mol Cell. 2005 Oct 28;20(2):301-12. doi: 10.1016/j.molcel.2005.09.014. Mol Cell. 2005. PMID: 16246731
-
TRAF6-mediated ubiquitination regulates nuclear translocation of NRIF, the p75 receptor interactor.EMBO J. 2005 Nov 16;24(22):3859-68. doi: 10.1038/sj.emboj.7600845. Epub 2005 Oct 27. EMBO J. 2005. PMID: 16252010 Free PMC article.
-
C-Cbl negatively regulates TRAF6-mediated NF-κB activation by promoting K48-linked polyubiquitination of TRAF6.Cell Mol Biol Lett. 2019 May 14;24:29. doi: 10.1186/s11658-019-0156-y. eCollection 2019. Cell Mol Biol Lett. 2019. PMID: 31123462 Free PMC article.
-
Signal integration and diversification through the p62 scaffold protein.Trends Biochem Sci. 2007 Feb;32(2):95-100. doi: 10.1016/j.tibs.2006.12.002. Epub 2006 Dec 15. Trends Biochem Sci. 2007. PMID: 17174552 Review.
-
Molecular interactions between neurotrophin receptors.Cell Tissue Res. 2001 Aug;305(2):229-38. doi: 10.1007/s004410100378. Cell Tissue Res. 2001. PMID: 11545260 Review.
Cited by
-
Remodeling without destruction: non-proteolytic ubiquitin chains in neural function and brain disorders.Mol Psychiatry. 2021 Jan;26(1):247-264. doi: 10.1038/s41380-020-0849-7. Epub 2020 Jul 24. Mol Psychiatry. 2021. PMID: 32709994 Free PMC article. Review.
-
Lysine 63-linked ubiquitination modulates mixed lineage kinase-3 interaction with JIP1 scaffold protein in cytokine-induced pancreatic β cell death.J Biol Chem. 2013 Jan 25;288(4):2428-40. doi: 10.1074/jbc.M112.425884. Epub 2012 Nov 21. J Biol Chem. 2013. PMID: 23172226 Free PMC article.
-
TRAF6 autoubiquitination-independent activation of the NFkappaB and MAPK pathways in response to IL-1 and RANKL.PLoS One. 2008;3(12):e4064. doi: 10.1371/journal.pone.0004064. Epub 2008 Dec 29. PLoS One. 2008. PMID: 19112497 Free PMC article.
-
Toxoplasma GRA15 limits parasite growth in IFNγ-activated fibroblasts through TRAF ubiquitin ligases.EMBO J. 2020 May 18;39(10):e103758. doi: 10.15252/embj.2019103758. Epub 2020 Apr 15. EMBO J. 2020. PMID: 32293748 Free PMC article.
-
Tumor biomarkers for diagnosis, prognosis and targeted therapy.Signal Transduct Target Ther. 2024 May 20;9(1):132. doi: 10.1038/s41392-024-01823-2. Signal Transduct Target Ther. 2024. PMID: 38763973 Free PMC article. Review.
References
-
- Moscat JM, Diaz-Meco MT, Wooten MW. Signal integration and diversification through the p62 scaffold protein. Trends Biochem Sci. 2007;32:95–100. - PubMed
-
- Geetha T, Wooten MW. Structure and functional properties of the ubiquitin binding protein p62. FEBS Lett. 2002;512:19–24. - PubMed
-
- Mueller TD, Feigon J. Solution structures of UBA domains reveal a conserved hydrophobic surface for protein-protein interactions. J. Mol. Biol. 2002;319:1243–1255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

