Glycation does not modify bovine serum albumin (BSA)-induced reduction of rat aortic relaxation: the response to glycated and nonglycated BSA is lost in metabolic syndrome
- PMID: 18458031
- PMCID: PMC2430009
- DOI: 10.1093/glycob/cwn034
Glycation does not modify bovine serum albumin (BSA)-induced reduction of rat aortic relaxation: the response to glycated and nonglycated BSA is lost in metabolic syndrome
Abstract
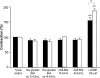
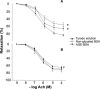
The effects of nonglycated bovine serum albumin (BSA) and advanced glycosylation end products of BSA (AGE-BSA) on vascular responses of control and metabolic syndrome (MS) rats characterized by hypertriglyceridemia, hypertension, hyperinsulinemia, and insulin resistance were studied. Albumin and in vitro prepared AGE-BSA have vascular effects; however, recent studies indicate that some effects of in vitro prepared AGEs are due to the conditions in which they were generated. We produced AGEs by incubating glucose with BSA for 60 days under sterile conditions in darkness and at 37 degrees C. To develop MS rats, male Wistar animals were given 30% sucrose in drinking water since weanling. Six month old animals were used. Blood pressure, insulin, triglycerides, and serum albumin were increased in MS rats. Contraction of aortic rings elicited with norepinephrine was stronger. There were no effects of nonglycated BSA or AGE-BSA on contractions in control or MS rats; however, both groups responded to L-NAME, an inhibitor of nitric oxide synthesis. Arterial relaxation induced using acetylcholine was smaller in MS rats. Nonglycated BSA and AGE-BSA significantly diminished relaxation in a 35% in the control group but the decrease was similar when using nonglycated BSA and AGE-BSA. This decrease was not present in the MS rats and was not due to increased RAGEs or altered biochemical characteristics of BSA. In conclusion, both BSA and AGE-BSA inhibit vascular relaxation in control artic rings. In MS rats the effect is lost possibly due to alterations in endothelial cells that are a consequence of the illness.
Figures


References
-
- Al-Abed Y, Kapurniotu A, Bucala R. Advanced glycation end products: Detection and reversal. Methods Enzymol. 1999;309:152–172. - PubMed
-
- Allen MT, Patterson SM. Hemoconcentration and stress: A review of physiological mechanisms and relevance for cardiovascular disease risk. Biol Psychol. 1995;41(1):1–27. - PubMed
-
- Baños G, Carvajal K, Cardoso G, Zamora J, Franco M. Vascular reactivity and effect of serum in a rat model of hypertriglyceridemia and hypertension. Am J Hypertens. 1997;10:379–388. - PubMed
-
- Baños G, Medina-Campos ON, Maldonado P, Zamora J, Pérez I, Pavón N, Pedraza-Chaverrí J. Antioxidant enzymes in hypertensive and hypertriglyceridemic rats: Effect of gender. Clin Exp Hypertens. 2005;1:45–57. - PubMed
-
- Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: A mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–822. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

