The assembly pathway of the mitochondrial carrier translocase involves four preprotein translocases
- PMID: 18458057
- PMCID: PMC2447139
- DOI: 10.1128/MCB.02216-07
The assembly pathway of the mitochondrial carrier translocase involves four preprotein translocases
Abstract
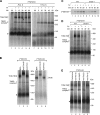
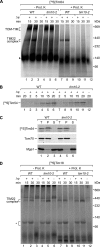
The mitochondrial inner membrane contains preprotein translocases that mediate insertion of hydrophobic proteins. Little is known about how the individual components of these inner membrane preprotein translocases combine to form multisubunit complexes. We have analyzed the assembly pathway of the three membrane-integral subunits Tim18, Tim22, and Tim54 of the twin-pore carrier translocase. Tim54 displayed the most complex pathway involving four preprotein translocases. The precursor is translocated across the intermembrane space in a supercomplex of outer and inner membrane translocases. The TIM10 complex, which translocates the precursor of Tim22 through the intermembrane space, functions in a new posttranslocational manner: in case of Tim54, it is required for the integration of Tim54 into the carrier translocase. Tim18, the function of which has been unknown so far, stimulates integration of Tim54 into the carrier translocase. We show that the carrier translocase is built via a modular process and that each subunit follows a different assembly route. Membrane insertion and assembly into the oligomeric complex are uncoupled for each precursor protein. We propose that the mitochondrial assembly machinery has adapted to the needs of each membrane-integral subunit and that the uncoupling of translocation and oligomerization is an important principle to ensure continuous import and assembly of protein complexes in a highly active membrane.
Figures

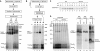
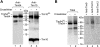
References
-
- Chacinska, A., M. Lind, A. E. Frazier, J. Dudek, C. Meisinger, A. Geissler, A. Sickmann, H. E. Meyer, K. N. Truscott, N. Pfanner, and P. Rehling. 2005. Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell 120817-829. - PubMed
-
- Dekker, P. J. T., F. Martin, A. C. Maarse, U. Bömer, H. Müller, B. Guiard, M. Meijer, J. Rassow, and N. Pfanner. 1997. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 165408-5419. - PMC - PubMed
-
- Dekker, P. J. T., H. Müller, J. Rassow, and N. Pfanner. 1996. Characterization of the preprotein translocase of the outer mitochondrial membrane by blue native electrophoresis. Biol. Chem. 377535-538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
