Chaperone control of the activity and specificity of the histone H3 acetyltransferase Rtt109
- PMID: 18458063
- PMCID: PMC2447148
- DOI: 10.1128/MCB.00182-08
Chaperone control of the activity and specificity of the histone H3 acetyltransferase Rtt109
Abstract
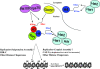
Acetylation of Saccharomyces cerevisiae histone H3 on K56 by the histone acetyltransferase (HAT) Rtt109 is important for repairing replication-associated lesions. Rtt109 purifies from yeast in complex with the histone chaperone Vps75, which stabilizes the HAT in vivo. A whole-genome screen to identify genes whose deletions have synthetic genetic interactions with rtt109Delta suggests Rtt109 has functions in addition to DNA repair. We show that in addition to its known H3-K56 acetylation activity, Rtt109 is also an H3-K9 HAT, and we show that Rtt109 and Gcn5 are the only H3-K9 HATs in vivo. Rtt109's H3-K9 acetylation activity in vitro is enhanced strongly by Vps75. Another histone chaperone, Asf1, and Vps75 are both required for acetylation of lysine 9 on H3 (H3-K9ac) in vivo by Rtt109, whereas H3-K56ac in vivo requires only Asf1. Asf1 also physically interacts with the nuclear Hat1/Hat2/Hif1 complex that acetylates H4-K5 and H4-K12. We suggest Asf1 is capable of assembling into chromatin H3-H4 dimers diacetylated on both H4-K5/12 and H3-K9/56.
Figures



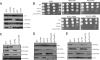

References
-
- Adkins, M. W., J. J. Carson, C. M. English, C. J. Ramey, and J. K. Tyler. 2007. The histone chaperone anti-silencing function 1 stimulates the acetylation of newly synthesized histone H3 in S-phase. J. Biol. Chem. 2821334-1340. - PubMed
-
- Adkins, M. W., and J. K. Tyler. 2004. The histone chaperone Asf1p mediates global chromatin disassembly in vivo. J. Biol. Chem. 27952069-52074. - PubMed
-
- Ai, X., and M. R. Parthun. 2004. The nuclear Hat1p/Hat2p complex: a molecular link between type B histone acetyltransferases and chromatin assembly. Mol. Cell 14195-205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
