Application of capillary electrophoresis mass spectrometry and liquid chromatography multiple-step tandem electrospray mass spectrometry to profile glycoform expression during Haemophilus influenzae pathogenesis in the chinchilla model of experimental otitis media
- PMID: 18458064
- PMCID: PMC2446737
- DOI: 10.1128/IAI.01710-07
Application of capillary electrophoresis mass spectrometry and liquid chromatography multiple-step tandem electrospray mass spectrometry to profile glycoform expression during Haemophilus influenzae pathogenesis in the chinchilla model of experimental otitis media
Abstract
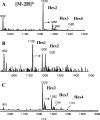
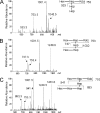
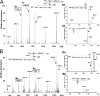
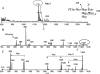
Otitis media caused by nontypeable Haemophilus influenzae (NTHi) is a common and recurrent bacterial infection of childhood. The structural variability and diversity of H. influenzae lipopolysaccharide (LPS) glycoforms are known to play a significant role in the commensal and disease-causing behavior of this pathogen. In this study, we determined LPS glycoform populations from NTHi strain 1003 during the course of experimental otitis media in the chinchilla model of infection by mass spectrometric techniques. Building on an established structural model of the major LPS glycoforms expressed by this NTHi strain in vitro (M. Månsson, W. Hood, J. Li, J. C. Richards, E. R. Moxon, and E. K. Schweda, Eur. J. Biochem. 269:808-818, 2002), minor isomeric glycoform populations were determined by liquid chromatography multiple-step tandem electrospray mass spectrometry (LC-ESI-MS(n)). Using capillary electrophoresis ESI-MS (CE-ESI-MS), we determined glycoform profiles for bacteria from direct middle ear fluid (MEF) samples. The LPS glycan profiles were essentially the same when the MEF samples of 7 of 10 animals were passaged on solid medium (chocolate agar). LC-ESI-MS(n) provided a sensitive method for determining the isomeric distribution of LPS glycoforms in MEF and passaged specimens. To investigate changes in LPS glycoform distribution during the course of infection, MEF samples were analyzed at 2, 5, and 9 days postinfection by CE-ESI-MS following minimal passage on chocolate agar. As previously observed, sialic acid-containing glycoforms were detected during the early stages of infection, but a trend toward more-truncated and less-complex LPS glycoforms that lacked sialic acid was found as disease progressed.
Figures







References
-
- Bakaletz, L. O. 2007. Bacterial biofilms in otitis media: evidence and relevance. Pediatr. Infect. Dis. J. 26S17-S19. - PubMed
-
- Bernstein, J. M., H. S. Faden, B. G. Loos, T. F. Murphy, and P. L. Ogra. 1992. Recurrent otitis media with non-typeable Haemophilus influenzae: the role of serum bactericidal antibody. Int. J. Pediatr. Otorhinolaryngol. 231-13. - PubMed
-
- Blakeney, A. B., and B. A. Stone. 1985. Methylation of carbohydrates with lithium methylsulphinyl carbanion. Carbohydr. Res. 140319-324.
-
- Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. A. Randle, A. Martin, Z. Li, R. Goldstein, E. K. Schweda, S. I. Pelton, J. C. Richards, and E. R. Moxon. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 1008898-8903. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

