Expression of CD1d and ligand-induced cytokine production are tissue specific in mucosal epithelia of the human lower reproductive tract
- PMID: 18458073
- PMCID: PMC2446716
- DOI: 10.1128/IAI.01672-07
Expression of CD1d and ligand-induced cytokine production are tissue specific in mucosal epithelia of the human lower reproductive tract
Abstract
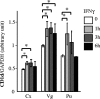
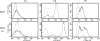
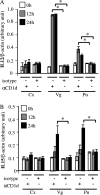
Mucosal epithelia of the human lower reproductive tract (vagina, cervix, and penile urethra) are exposed to sexually transmitted microbes, including Chlamydia trachomatis. The in vivo susceptibility of each tissue type to infection with C. trachomatis is quite distinct. CD1d is expressed on the surface of antigen-presenting cells, including mucosal epithelial cells, and interacts specifically with invariant NKT cells. Invariant NKT cells play a role in both innate and adaptive immune responses to microbes. Here we assessed CD1d expression in normal reproductive tissues by using immunohistochemistry. Immortalized epithelial cell lines from the human lower reproductive tract (vagina, endocervix, and penile urethra) were examined for CD1d expression and for ligand-induced cytokine production induced by CD1d cross-linking. CD1d expression in normal tissue was strong in the vagina but weak in the endocervix and penile urethra. Gamma interferon exposure induced CD1d transcription in all of the cell types studied, with the strongest induction in vaginal cells. Flow cytometry revealed cell surface expression of CD1d in vaginal and penile urethral epithelial cells but not in endocervical cells. Ligation of surface-expressed CD1d by monoclonal antibody cross-linking promoted interleukin-12 (IL-12) and IL-15, but not IL-10, production in vaginal and penile urethral cells. No induction was demonstrated in endocervical cells. CD1d-mediated cytokine production in penile urethral cells was abrogated by C. trachomatis infection. Basal deficiency in CD1d-mediated immune responsiveness may result in susceptibility to sexually transmitted agents. Decreased CD1d-mediated signaling may help C. trachomatis evade detection by innate immune cells.
Figures

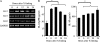
References
-
- Behar, S. M., and S. A. Porcelli. 2007. CD1-restricted T cells in host defense to infectious diseases. Curr. Top. Microbiol. Immunol. 314215-250. - PubMed
-
- Bilenki, L., S. Wang, J. Yang, Y. Fan, A. G. Joyee, and X. Yang. 2005. NK T cell activation promotes Chlamydia trachomatis infection in vivo. J. Immunol. 1753197-3206. - PubMed
-
- Blumberg, R. S., C. Terhorst, P. Bleicher, F. V. McDermott, C. H. Allan, S. B. Landau, J. S. Trier, and S. P. Balk. 1991. Expression of a nonpolymorphic MHC class1-like molecule, CD1D, by human intestinal epithelial cells. J. Immunol. 1472518-2524. - PubMed
-
- Bonish, B., D. Jullien, Y. Dutronc, B. B. Huang, R. Modlin, F. M. Spada, S. A. Porcelli, and B. J. Nickoloff. 2000. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-γ production by NK-T cells. J. Immunol. 1654076-4085. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

