Mechanisms of ligand transfer by the hepatic tocopherol transfer protein
- PMID: 18458085
- PMCID: PMC2440614
- DOI: 10.1074/jbc.M800121200
Mechanisms of ligand transfer by the hepatic tocopherol transfer protein
Abstract
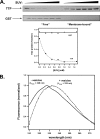
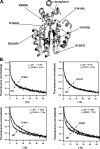
alpha-Tocopherol is a member of the vitamin E family that functions as the principal fat-soluble antioxidant in vertebrates. Body-wide distribution of tocopherol is regulated by the hepatic alpha-tocopherol transfer protein (alphaTTP), which stimulates secretion of the vitamin from hepatocytes to circulating lipoproteins. This biological activity of alphaTTP is thought to stem from its ability to facilitate the transfer of vitamin E between membranes, but the mechanism by which the protein exerts this activity remains poorly understood. Using a fluorescence energy transfer methodology, we found that the rate of tocopherol transfer from lipid vesicles to alphaTTP increases with increasing alphaTTP concentration. This concentration dependence indicates that ligand transfer by alphaTTP involves direct protein-membrane interaction. In support of this notion, equilibrium analyses employing filtration, dual polarization interferometry, and tryptophan fluorescence demonstrated the presence of a stable alphaTTP-bilayer complex. The physical association of alphaTTP with membranes is markedly sensitive to the presence of vitamin E in the bilayer. Some naturally occurring mutations in alphaTTP that cause the hereditary disorder ataxia with vitamin E deficiency diminish the effect of tocopherol on the protein-membrane association, suggesting a possible mechanism for the accompanying pathology.
Figures




References
-
- Traber, M. G., and Kayden, H. J. (1989) Am. J. Clin. Nutr. 49 517–526 - PubMed
-
- Behrens, W. A., and Madere, R. (1986) J. Am. Coll. Nutr. 5 91–96 - PubMed
-
- Sontag, T. J., and Parker, R. S. (2002) J. Biol. Chem. 277 25290–25296 - PubMed
-
- Swanson, J. E., Ben, R. N., Burton, G. W., and Parker, R. S. (1999) J. Lipid Res. 40 665–671 - PubMed
-
- Panagabko, C., Morley, S., Hernandez, M., Cassolato, P., Gordon, H., Parsons, R., Manor, D., and Atkinson, J. (2003) Biochemistry 42 6467–6474 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

