Endogenous hydrogen peroxide regulates glutathione redox via nuclear factor erythroid 2-related factor 2 downstream of phosphatidylinositol 3-kinase during muscle differentiation
- PMID: 18458092
- PMCID: PMC2408414
- DOI: 10.2353/ajpath.2008.070429
Endogenous hydrogen peroxide regulates glutathione redox via nuclear factor erythroid 2-related factor 2 downstream of phosphatidylinositol 3-kinase during muscle differentiation
Abstract
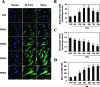
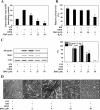
We reported previously that endogenous reactive oxygen species (ROS) function as myogenic signaling molecules. It has also been determined that excess ROS induce electrophile-response element (EpRE)-driven gene expression via activation of nuclear factor erythroid 2-related factor 2 (Nrf2). Nonetheless, the relationship between the metabolism of ROS (eg, H(2)O(2)) through glutathione (GSH) up-regulation, GSH-dependent reduction of H(2)O(2), and Nrf2-dependent gene regulation is not well established. Therefore, we attempted to determine whether H(2)O(2) controls the intracellular GSH redox state via the Nrf2-glutamate-cysteine ligase (GCL)/glutathione reductase (GR)-GSH signaling pathway. In our experiments, enhanced H(2)O(2) generation was accompanied by an increase in both total GSH levels and the GSH/GSSG ratio during muscle differentiation. Both GCL and GR transcriptional expression levels were markedly increased during muscle differentiation but reduced by catalase treatment. Nrf2 protein expression and nuclear translocation increased during myogenesis. The inhibition of GCL, GR, and Nrf2 both by inhibitors and by RNA interference blocked muscle differentiation. Phosphatidylinositol 3-kinase regulated the expression of the GCL C (a catalytic subunit) and GR genes via the induction of Nrf2 nuclear translocation and expression. In conclusion, endogenous H(2)O(2) generated during muscle differentiation not only functions as a signaling molecule, but also regulates the GSH redox state via activation of the Nrf2-GCL/GR-GSH signaling pathway downstream of phosphatidylinositol 3-kinase.
Figures






References
-
- Lynch GS, Schertzer JD, Ryall JG. Therapeutic approaches for muscle wasting disorders. Pharmacol Ther. 2007;113:461–487. - PubMed
-
- Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol. 2000;218:115–124. - PubMed
-
- Lim MJ, Choi KJ, Ding Y, Kim JH, Kim BS, Kim YH, Lee J, Choe W, Kang I, Ha J, Yoon KS, Kim SS. RhoA/Rho kinase blocks muscle differentiation via serine phosphorylation of insulin receptor substrate-1 and -2. Mol Endocrinol. 2007;21:2282–2293. - PubMed
-
- Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem. 2005;280:2737–2744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

