Expression and maintenance of mitochondrial DNA: new insights into human disease pathology
- PMID: 18458094
- PMCID: PMC2408405
- DOI: 10.2353/ajpath.2008.071163
Expression and maintenance of mitochondrial DNA: new insights into human disease pathology
Abstract
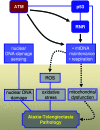
Mitochondria are central players in cellular energy metabolism and, consequently, defects in their function result in many characterized metabolic diseases. Critical for their function is mitochondrial DNA (mtDNA), which encodes subunits of the oxidative phosphorylation complexes essential for cellular respiration and ATP production. Expression, replication, and maintenance of mtDNA require factors encoded by nuclear genes. These include not only the primary machinery involved (eg, transcription and replication components) but also those in signaling pathways that mediate or sense alterations in mitochondrial function in accord with changing cellular needs or environmental conditions. Mutations in these contribute to human disease pathology by mechanisms that are being revealed at an unprecedented rate. As I will discuss herein, the basic protein machinery required for transcription initiation in human mitochondria has been elucidated after the discovery of two multifunctional mitochondrial transcription factors, h-mtTFB1 and h-mtTFB2, that are also rRNA methyltransferases. In addition, involvement of the ataxia-telangiectasia mutated (ATM) and target of rapamycin (TOR) signaling pathways in regulating mitochondrial homeostasis and gene expression has also recently been uncovered. These advancements embody the current mitochondrial research landscape, which can be described as exploding with discoveries of previously unanticipated roles for mitochondria in human disease and aging.
Figures

References
-
- Bonawitz ND, Clayton DA, Shadel GS. Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol Cell. 2006;24:813–825. - PubMed
-
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. - PubMed
-
- Bonawitz ND, Shadel GS. Rethinking the mitochondrial theory of aging: the role of mitochondrial gene expression in lifespan determination. Cell Cycle. 2007;6:1574–1578. - PubMed
-
- Kujoth GC, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations and apoptosis in mammalian aging. Cancer Res. 2006;66:7386–7389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

