Altered dosage and mislocalization of histone H3 and Cse4p lead to chromosome loss in Saccharomyces cerevisiae
- PMID: 18458100
- PMCID: PMC2390605
- DOI: 10.1534/genetics.108.088518
Altered dosage and mislocalization of histone H3 and Cse4p lead to chromosome loss in Saccharomyces cerevisiae
Abstract
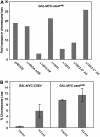
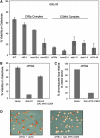
Cse4p is an essential histone H3 variant in Saccharomyces cerevisiae that defines centromere identity and is required for proper segregation of chromosomes. In this study, we investigated phenotypic consequences of Cse4p mislocalization and increased dosage of histone H3 and Cse4p, and established a direct link between histone stoichiometry, mislocalization of Cse4p, and chromosome segregation. Overexpression of the stable Cse4p mutant, cse4(K16R), resulted in its mislocalization, increased association with chromatin, and a high rate of chromosome loss, all of which were suppressed by constitutive expression of histone H3 (delta 16H3). We determined that delta 16H3 did not lead to increased chromosome loss; however, increasing the dosage of histone H3 (GALH3) resulted in significant chromosome loss due to reduced levels of centromere (CEN)-associated Cse4p and synthetic dosage lethality (SDL) in kinetochore mutants. These phenotypes were suppressed by GALCSE4. We conclude that the chromosome missegregation of GALcse4(K16R) and GALH3 strains is due to mislocalization and a functionally compromised kinetochore, respectively. Suppression of these phenotypes by histone delta 16H3 and GALCSE4 supports the conclusion that proper stoichiometry affects the localization of histone H3 and Cse4p and is thus essential for accurate chromosome segregation.
Figures




References
-
- Bloom, K. S., and J. Carbon, 1982. Yeast centromere DNA is in a unique and highly ordered structure in chromosomes and small circular minichromosomes. Cell 29 305–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

