Hsp90/Hsp70 chaperone machine regulation of the Saccharomyces MAL-activator as determined in vivo using noninducible and constitutive mutant alleles
- PMID: 18458105
- PMCID: PMC2390613
- DOI: 10.1534/genetics.107.084921
Hsp90/Hsp70 chaperone machine regulation of the Saccharomyces MAL-activator as determined in vivo using noninducible and constitutive mutant alleles
Abstract
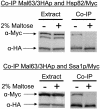
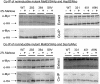
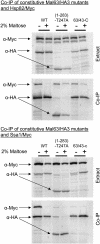
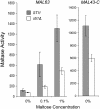
The Hsp90/Hsp70 chaperone machine is an essential regulator of cell growth and division. It is required for activation of select client proteins, chiefly protein kinases and transcription activators and thus plays a major role in regulating intracellular signaling and gene expression. This report demonstrates, in vivo, the association of the Saccharomyces cerevisiae maltose-responsive transcription activator Mal63 (MAL-activator) with the yeast Hsp70 (Ssa1), Hsp90 (Hsp82), and Hop (Sti1) homologs, using a collection of inducible, constitutive, and noninducible alleles. Each class of mutant activator forms a distinctly different stable multichaperone complex in the absence of maltose. Inducible Mal63p associates with Ssa1, Hsp82, and Sti1 and is released in the presence of maltose. Noninducible mal63 mutant proteins bind to Ssa1 alone and do not stably associate with Hsp82 or Sti1. Constitutive MAL-activators bind well to Hsp82 and poorly to Ssa1 and Sti1, but deletion of STI1 restores Ssa1 binding. Taken together, Mal63p regulation requires the formation of Hsp90/Hsp70 subcomplexes comparable to, yet distinct from those observed with previously characterized Hsp90 clients including glucocorticoid receptor and yeast Hap1p. Thus, comparative studies of different client proteins highlight functional diversity in the operation of the Hsp90/Hsp70 chaperone machine.
Figures





Similar articles
-
Hsp90 cochaperone Aha1 is a negative regulator of the Saccharomyces MAL activator and acts early in the chaperone activation pathway.J Biol Chem. 2010 Apr 30;285(18):13850-62. doi: 10.1074/jbc.M109.040600. Epub 2010 Feb 22. J Biol Chem. 2010. PMID: 20177068 Free PMC article.
-
The Hsp90 molecular chaperone complex regulates maltose induction and stability of the Saccharomyces MAL gene transcription activator Mal63p.J Biol Chem. 2003 Nov 28;278(48):47441-8. doi: 10.1074/jbc.M309536200. Epub 2003 Sep 18. J Biol Chem. 2003. PMID: 14500708
-
Functional and physical interaction between yeast Hsp90 and Hsp70.Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):E2210-E2219. doi: 10.1073/pnas.1719969115. Epub 2018 Feb 20. Proc Natl Acad Sci U S A. 2018. PMID: 29463764 Free PMC article.
-
New insights into Sti1/Hop's cochaperone function highlight the complexity of proteostatic regulation.FEBS J. 2025 Jul;292(14):3629-3633. doi: 10.1111/febs.70108. Epub 2025 Apr 21. FEBS J. 2025. PMID: 40259657 Free PMC article. Review.
-
A unique mechanism of chaperone action: heme regulation of Hap1 activity involves separate control of repression and activation.Protein Pept Lett. 2009;16(6):642-9. doi: 10.2174/092986609788490113. Protein Pept Lett. 2009. PMID: 19519523 Review.
Cited by
-
Hsp90 cochaperone Aha1 is a negative regulator of the Saccharomyces MAL activator and acts early in the chaperone activation pathway.J Biol Chem. 2010 Apr 30;285(18):13850-62. doi: 10.1074/jbc.M109.040600. Epub 2010 Feb 22. J Biol Chem. 2010. PMID: 20177068 Free PMC article.
-
Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system.Microbiol Mol Biol Rev. 2012 Jun;76(2):115-58. doi: 10.1128/MMBR.05018-11. Microbiol Mol Biol Rev. 2012. PMID: 22688810 Free PMC article. Review.
-
Regulations of sugar transporters: insights from yeast.Curr Genet. 2013 May;59(1-2):1-31. doi: 10.1007/s00294-013-0388-8. Epub 2013 Mar 1. Curr Genet. 2013. PMID: 23455612 Review.
-
Transcriptional regulation in Saccharomyces cerevisiae: transcription factor regulation and function, mechanisms of initiation, and roles of activators and coactivators.Genetics. 2011 Nov;189(3):705-36. doi: 10.1534/genetics.111.127019. Genetics. 2011. PMID: 22084422 Free PMC article. Review.
-
Hsp90 Maintains Proteostasis of the Galactose Utilization Pathway To Prevent Cell Lethality.Mol Cell Biol. 2016 Apr 15;36(9):1412-24. doi: 10.1128/MCB.01064-15. Print 2016 May. Mol Cell Biol. 2016. PMID: 26951197 Free PMC article.
References
-
- Bali, M., B. Zhang, K. A. Morano and C. A. Michels, 2003. The Hsp90 molecular chaperone complex regulates maltose induction and stability of the Saccharomyces MAL gene transcription activator Mal63p. J. Biol. Chem. 278 47441–47448. - PubMed
-
- Brinker, A., C. Scheufler, F. Von Der Mulbe, B. Fleckenstein, C. Herrmann et al., 2002. Ligand discrimination by TPR domains. Relevance and selectivity of EEVD-recognition in Hsp70 × Hop × Hsp90 complexes. J. Biol. Chem. 277 19265–19275. - PubMed
-
- Bukau, B., J. Weissman and A. Horwich, 2006. Molecular chaperones and protein quality control. Cell 125 443–451. - PubMed
-
- Caplan, A. J., A. K. Mandal and M. A. Theodoraki, 2007. Molecular chaperones and protein kinase quality control. Trends Cell Biol. 17 87–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

