Degradation of functional triose phosphate isomerase protein underlies sugarkill pathology
- PMID: 18458110
- PMCID: PMC2429879
- DOI: 10.1534/genetics.108.087551
Degradation of functional triose phosphate isomerase protein underlies sugarkill pathology
Abstract
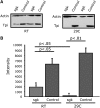
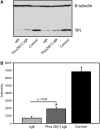
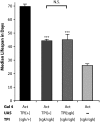
Triose phosphate isomerase (TPI) deficiency glycolytic enzymopathy is a progressive neurodegenerative condition that remains poorly understood. The disease is caused exclusively by specific missense mutations affecting the TPI protein and clinically features hemolytic anemia, adult-onset neurological impairment, degeneration, and reduced longevity. TPI has a well-characterized role in glycolysis, catalyzing the isomerization of dihydroxyacetone phosphate (DHAP) to glyceraldehyde-3-phosphate (G3P); however, little is known mechanistically about the pathogenesis associated with specific recessive mutations that cause progressive neurodegeneration. Here, we describe key aspects of TPI pathogenesis identified using the TPI(sugarkill) mutation, a Drosophila model of human TPI deficiency. Specifically, we demonstrate that the mutant protein is expressed, capable of forming a homodimer, and is functional. However, the mutant protein is degraded by the 20S proteasome core leading to loss-of-function pathogenesis.
Figures
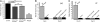


Similar articles
-
Hsp70- and Hsp90-mediated proteasomal degradation underlies TPI sugarkill pathogenesis in Drosophila.Neurobiol Dis. 2010 Dec;40(3):676-83. doi: 10.1016/j.nbd.2010.08.011. Epub 2010 Aug 19. Neurobiol Dis. 2010. PMID: 20727972 Free PMC article.
-
Evidence of a triosephosphate isomerase non-catalytic function crucial to behavior and longevity.J Cell Sci. 2013 Jul 15;126(Pt 14):3151-8. doi: 10.1242/jcs.124586. Epub 2013 May 2. J Cell Sci. 2013. PMID: 23641070 Free PMC article.
-
Drosophila model of human inherited triosephosphate isomerase deficiency glycolytic enzymopathy.Genetics. 2006 Nov;174(3):1237-46. doi: 10.1534/genetics.106.063206. Epub 2006 Sep 15. Genetics. 2006. PMID: 16980388 Free PMC article.
-
Triosephosphate isomerase deficiency: facts and doubts.IUBMB Life. 2006 Dec;58(12):703-15. doi: 10.1080/15216540601115960. IUBMB Life. 2006. PMID: 17424909 Review.
-
Newly discovered roles of triosephosphate isomerase including functions within the nucleus.Mol Med. 2023 Jan 31;29(1):18. doi: 10.1186/s10020-023-00612-x. Mol Med. 2023. PMID: 36721084 Free PMC article. Review.
Cited by
-
Triose-phosphate isomerase deficiency is associated with a dysregulation of synaptic vesicle recycling in Drosophila melanogaster.Front Synaptic Neurosci. 2023 Feb 28;15:1124061. doi: 10.3389/fnsyn.2023.1124061. eCollection 2023. Front Synaptic Neurosci. 2023. PMID: 36926383 Free PMC article.
-
Triosephosphate isomerase I170V alters catalytic site, enhances stability and induces pathology in a Drosophila model of TPI deficiency.Biochim Biophys Acta. 2015 Jan;1852(1):61-9. doi: 10.1016/j.bbadis.2014.10.010. Epub 2014 Oct 16. Biochim Biophys Acta. 2015. PMID: 25463631 Free PMC article.
-
Identification of protein quality control regulators using a Drosophila model of TPI deficiency.Neurobiol Dis. 2021 May;152:105299. doi: 10.1016/j.nbd.2021.105299. Epub 2021 Feb 15. Neurobiol Dis. 2021. PMID: 33600953 Free PMC article.
-
Hsp70- and Hsp90-mediated proteasomal degradation underlies TPI sugarkill pathogenesis in Drosophila.Neurobiol Dis. 2010 Dec;40(3):676-83. doi: 10.1016/j.nbd.2010.08.011. Epub 2010 Aug 19. Neurobiol Dis. 2010. PMID: 20727972 Free PMC article.
-
Sequencing and genotypic analysis of the triosephosphate isomerase (TPI1) locus in a large sample of long-lived Germans.BMC Genet. 2008 May 29;9:38. doi: 10.1186/1471-2156-9-38. BMC Genet. 2008. PMID: 18510744 Free PMC article.
References
-
- Ahmed, N., S. Battah, N. Karachalias, R. Babaei-Jadidi, M. Horanyi et al., 2003. Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochim. Biophys. Acta 1639 121–132. - PubMed
-
- Arya, R., M. R. Lalloz, A. J. Bellingham and D. M. Layton, 1997. Evidence for founder effect of the Glu104Asp substitution and identification of new mutations in triosephosphate isomerase deficiency. Hum. Mutat. 10 290–294. - PubMed
-
- Ationu, A., A. Humphries, M. R. Lalloz, R. Arya, B. Wild et al., 1999. Reversal of metabolic block in glycolysis by enzyme replacement in triosephosphate isomerase-deficient cells. Blood 94 3193–3198. - PubMed
-
- Barral, J. M., S. A. Broadley, G. Schaffar and F. U. Hartl, 2004. Roles of molecular chaperones in protein misfolding diseases. Semin. Cell Dev. Biol. 15 17–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

