Palmitate impairs and eicosapentaenoate restores insulin secretion through regulation of SREBP-1c in pancreatic islets
- PMID: 18458149
- PMCID: PMC2518489
- DOI: 10.2337/db06-1806
Palmitate impairs and eicosapentaenoate restores insulin secretion through regulation of SREBP-1c in pancreatic islets
Abstract
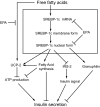
Objective: Chronic exposure to fatty acids causes beta-cell failure, often referred to as lipotoxicity. We investigated its mechanisms, focusing on contribution of SREBP-1c, a key transcription factor for lipogenesis.
Research design and methods: We studied in vitro and in vivo effects of saturated and polyunsaturated acids on insulin secretion, insulin signaling, and expression of genes involved in beta-cell functions. Pancreatic islets isolated from C57BL/6 control and SREBP-1-null mice and adenoviral gene delivery or knockdown systems of related genes were used.
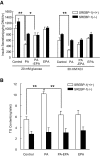
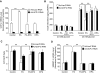
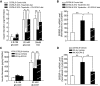
Results: Incubation of C57BL/6 islets with palmitate caused inhibition of both glucose- and potassium-stimulated insulin secretion, but addition of eicosapentaenoate (EPA) restored both inhibitions. Concomitantly, palmitate activated and EPA abolished both mRNA and nuclear protein of SREBP-1c, accompanied by reciprocal changes of SREBP-1c target genes such as insulin receptor substrate-2 (IRS-2) and granuphilin. These palmitate-EPA effects on insulin secretion were abolished in SREBP-1-null islets. Suppression of IRS-2/Akt pathway could be a part of the downstream mechanism for the SREBP-1c-mediated insulin secretion defect because adenoviral constitutively active Akt compensated it. Uncoupling protein-2 (UCP-2) also plays a crucial role in the palmitate inhibition of insulin secretion, as confirmed by knockdown experiments, but SREBP-1c contribution to UCP-2 regulation was partial. The palmitate-EPA regulation of insulin secretion was similarly observed in islets from C57BL/6 mice pretreated with dietary manipulations. Furthermore, administration of EPA to diabetic KK-Ay mice ameliorated impairment of insulin secretion in their islets.
Conclusions: SREBP-1c plays a dominant role in palmitate-mediated insulin secretion defect, and EPA prevents it through SREBP-1c inhibition, implicating a therapeutic potential for treating diabetes related to lipotoxicity.
Figures




Similar articles
-
Sterol regulatory element-binding protein-1c and pancreatic beta-cell dysfunction.Diabetes Obes Metab. 2007 Nov;9 Suppl 2:133-9. doi: 10.1111/j.1463-1326.2007.00779.x. Diabetes Obes Metab. 2007. PMID: 17919187
-
Long-Term Exposure of Pancreatic β-Cells to Palmitate Results in SREBP-1C-Dependent Decreases in GLP-1 Receptor Signaling via CREB and AKT and Insulin Secretory Response.Endocrinology. 2016 Jun;157(6):2243-58. doi: 10.1210/en.2015-2003. Epub 2016 Apr 1. Endocrinology. 2016. PMID: 27035653
-
Granuphilin is activated by SREBP-1c and involved in impaired insulin secretion in diabetic mice.Cell Metab. 2006 Aug;4(2):143-54. doi: 10.1016/j.cmet.2006.06.009. Cell Metab. 2006. PMID: 16890542
-
SREBP-1c and TFE3, energy transcription factors that regulate hepatic insulin signaling.J Mol Med (Berl). 2007 May;85(5):437-44. doi: 10.1007/s00109-007-0158-5. Epub 2007 Feb 6. J Mol Med (Berl). 2007. PMID: 17279346 Review.
-
Genetic manipulations of fatty acid metabolism in beta-cells are associated with dysregulated insulin secretion.Diabetes. 2002 Dec;51 Suppl 3:S414-20. doi: 10.2337/diabetes.51.2007.s414. Diabetes. 2002. PMID: 12475784 Review.
Cited by
-
Epigallocatechin gallate attenuated high glucose-induced pancreatic beta cell dysfunction by modulating DRP1-mediated mitochondrial apoptosis pathways.Sci Rep. 2024 Jul 22;14(1):16809. doi: 10.1038/s41598-024-67867-0. Sci Rep. 2024. PMID: 39039202 Free PMC article.
-
Myostatin induces insulin resistance via Casitas B-lineage lymphoma b (Cblb)-mediated degradation of insulin receptor substrate 1 (IRS1) protein in response to high calorie diet intake.J Biol Chem. 2014 Mar 14;289(11):7654-70. doi: 10.1074/jbc.M113.529925. Epub 2014 Jan 22. J Biol Chem. 2014. Retraction in: J Biol Chem. 2016 Jul 1;291(27):14392. doi: 10.1074/jbc.A113.529925. PMID: 24451368 Free PMC article. Retracted.
-
RNA-binding protein HuD reduces triglyceride production in pancreatic β cells by enhancing the expression of insulin-induced gene 1.Biochim Biophys Acta. 2016 Apr;1859(4):675-85. doi: 10.1016/j.bbagrm.2016.02.017. Epub 2016 Mar 3. Biochim Biophys Acta. 2016. PMID: 26945853 Free PMC article.
-
Susceptibility of pancreatic beta cells to fatty acids is regulated by LXR/PPARalpha-dependent stearoyl-coenzyme A desaturase.PLoS One. 2009 Sep 29;4(9):e7266. doi: 10.1371/journal.pone.0007266. PLoS One. 2009. PMID: 19787047 Free PMC article.
-
Lipotoxicity and β-Cell Failure in Type 2 Diabetes: Oxidative Stress Linked to NADPH Oxidase and ER Stress.Cells. 2021 Nov 26;10(12):3328. doi: 10.3390/cells10123328. Cells. 2021. PMID: 34943836 Free PMC article. Review.
References
-
- Kahn SE: Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 86 :4047 –4058,2001 - PubMed
-
- Weir GC, Bonner-Weir S: Five stages of evolving β-cell dysfunction during progression to diabetes. Diabetes 53 (Suppl. 3):S16 –S21,2004 - PubMed
-
- Stumvoll M, Goldstein BJ, van Haeften TW: Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365 :1333 –1346,2005 - PubMed
-
- Unger RH: Lipotoxicity in the pathogenesis of obesity-dependent NIDDM: genetic and clinical implications. Diabetes 44 :863 –870,1995 - PubMed
-
- Boden G, Shulman GI: Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 32 (Suppl. 3):14 –23,2002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

