Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling: role of CD14 and CD36
- PMID: 18458151
- PMCID: PMC3178504
- DOI: 10.1189/jlb.0907656
Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling: role of CD14 and CD36
Abstract
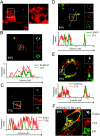
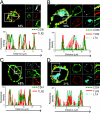
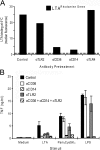
Lipoteichoic acid (LTA) is a central inducer of inflammatory responses caused by Gram-positive bacteria, such as Staphylococcus aureus, via activation of TLR2. Localization of TLR2 in relation to its coreceptors may be important for function. This study explores the signaling, uptake, and trafficking pattern of LTA in relation to expression of TLR2 and its coreceptors CD36 and CD14 in human monocytes. We found TLR2 expressed in early endosomes, late endosomes/lysosomes, and in Rab-11-positive compartments but not in the Golgi apparatus or endoplasmic reticulum (ER). Rapid internalization of fluorescently labeled LTA was observed in human monocytes, colocalizing with markers for early and late endosomes, lysosomes, ER, and Golgi network. Blocking CD14 and CD36 with antibodies inhibited LTA binding and LTA-induced TNF release from monocytes, emphasizing an important role for both molecules as coreceptors for TLR2. Importantly, blocking CD36 did not affect TNF release induced by N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2R,S)-propyl]-(R)-cysteinyl-seryl-(lysyl)3-lysine or LPS. Expression of CD14 markedly enhanced LTA binding to the plasma membrane and also enhanced NF-kappaB activation. LTA internalization, but not NF-kappaB activation, was inhibited in Dynamin-I K44A dominant-negative transfectants, suggesting that LTA is internalized by receptor-mediated endocytosis but that internalization is not required for signaling. In fact, immobilizing LTA and thereby inhibiting internalization resulted in enhanced TNF release from monocytes. Our results suggest that LTA signaling preferentially occurs at the plasma membrane, is independent of internalization, and is facilitated by CD36 and CD14 as coreceptors for TLR2.
Figures


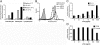


References
-
- Janeway C. A., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. - PubMed
-
- Medzhitov R., Janeway C. A., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. - PubMed
-
- Marshall C., Kossmann T., Wesselingh S., Spelman D. Methicillin-resistant Staphylococcus aureus and beyond: what’s new in the world of the golden staph? ANZ J Surg. 2004;74:465–469. - PubMed
-
- Raetz C. R., Brozek K. A., Clementz T., Coleman J. D., Galloway S. M., Golenbock D. T., Hampton R. Y. Gram-negative endotoxin: a biologically active lipid. Cold Spring Harb Symp Quant Biol. 1988;53:973–982. - PubMed
-
- Raetz C. R., Ulevitch R. J., Wright S. D., Sibley C. H., Ding A., Nathan C. F. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J. 1991;5:2652–2660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

