Electric field-induced astrocyte alignment directs neurite outgrowth
- PMID: 18458757
- PMCID: PMC2367316
- DOI: 10.1017/S1740925X0600010X
Electric field-induced astrocyte alignment directs neurite outgrowth
Abstract
The extension and directionality of neurite outgrowth are key to achieving successful target connections during both CNS development and during the re-establishment of connections lost after neural trauma. The degree of axonal elongation depends, in large part, on the spatial arrangement of astrocytic processes rich in growth-promoting proteins. Because astrocytes in culture align their processes on exposure to an electrical field of physiological strength, we sought to determine the extent to which aligned astrocytes affect neurite outgrowth. To this end, dorsal root ganglia cells were seeded onto cultured rat astrocytes that were pre-aligned by exposure to an electric field of physiological strength (500 mV mm(-1)). Using confocal microscopy and digital image analysis, we found that neurite outgrowth at 24 hours and at 48 hours is enhanced significantly and directed consistently along the aligned astrocyte processes. Moreover, this directed neurite outgrowth is maintained when grown on fixed, aligned astrocytes. Collectively, these results indicate that endogenous electric fields present within the developing CNS might act to align astrocyte processes, which can promote and direct neurite growth. Furthermore, these results demonstrate a simple method to produce an aligned cellular substrate, which might be used to direct regenerating neurites.
Keywords: Astrocytes; DRG; alignment; electric; neurite.
Figures

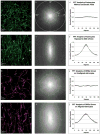

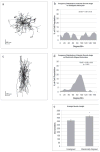
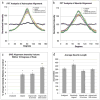
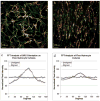
References
-
- Bandtlow CE, Schwab ME. NI-35/250/nogo-a: a neurite growth inhibitor restricting structural plasticity and regeneration of nerve fibers in the adult vertebrate CNS. Glia. 2000;29:175–181. - PubMed
-
- Biran R, Noble MD, Tresco PA. Directed nerve outgrowth is enhanced by engineered glial substrates. Experimental Neurology. 2003;184:141–152. - PubMed
-
- Borgens RB, Shi R, Mohr TJ, Jaeger CB. Mammalian cortical astrocytes align themselves in a physiological voltage gradient. Experimental Neurology. 1994;128:41–49. - PubMed
-
- Borgens RB, Vanable JW, Jr, Jaffe LF. Reduction of sodium dependent stump currents disturbs urodele limb regeneration. Journal of Experimental Zoology. 1979;209:377–386. - PubMed
-
- Cameron RS, Rakic P. Glial cell lineage in the cerebral cortex: a review and synthesis. Glia. 1991;4:124–137. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
