Protein backbone motions viewed by intraresidue and sequential HN-Halpha residual dipolar couplings
- PMID: 18458825
- PMCID: PMC2587141
- DOI: 10.1007/s10858-008-9237-3
Protein backbone motions viewed by intraresidue and sequential HN-Halpha residual dipolar couplings
Abstract
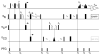
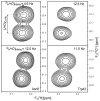
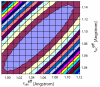
Triple resonance E.COSY-based techniques were used to measure intra-residue and sequential H(N)-H(alpha) residual dipolar couplings (RDCs) for the third IgG-binding domain of protein G (GB3), aligned in Pf1 medium. Measurements closely correlate with values predicted on the basis of an NMR structure, previously determined on the basis of a large number of one-bond backbone RDCs measured in five alignment media. However, in particular the sequential H(N)-H(alpha) RDCs are smaller than predicted for a static structure, suggesting a degree of motion for these internuclear vectors that exceeds that of the backbone amide N-H vectors. Of all experimentally determined GB3 structures available, the best correlation between experimental (1)H-(1)H couplings is observed for a GB3 ensemble, previously derived to generate a realistic picture of the conformational space sampled by GB3 (Clore and Schwieters, J Mol Biol 355:879-886, 2006). However, for both NMR and X-ray-derived structures the (1)H-(1)H couplings are found to be systematically smaller than expected on the basis of alignment tensors derived from (15)N-(1)H amide RDCs, assuming librationally corrected N-H bond lengths of 1.041 A.
Figures



References
-
- Bax A, Kontaxis G, Tjandra N. Dipolar couplings in macromolecular structure determination. Meth Enzymol. 2001;339:127–174. - PubMed
-
- Biamonti C, Rios CB, Lyons BA, Montelione GT. Multidimensional NMR experiments and analysis techniques for determining homo- and heteronuclear scalar coupling constants in proteins and nucleic acids. Advances in Biophysical Chemistry. 1994;4:51–120.
-
- Bolon PJ, Prestegard JH. COSY cross-peaks from H-1-H-1 dipolar couplings in NMR spectra of field oriented oligosaccharides. J Am Chem Soc. 1998;120:9366–9367.
-
- Cai ML, Wang H, Olejniczak ET, Meadows RP, Gunasekera AH, Xu N, Fesik SW. Accurate measurement of H-N-H-alpha residual dipolar couplings in proteins. J Magn Reson. 1999;139:451–453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

