Coordination of eye and head components of movements evoked by stimulation of the paramedian pontine reticular formation
- PMID: 18458891
- PMCID: PMC3655330
- DOI: 10.1007/s00221-008-1401-1
Coordination of eye and head components of movements evoked by stimulation of the paramedian pontine reticular formation
Abstract
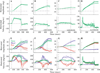
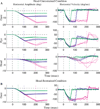
Constant frequency microstimulation of the paramedian pontine reticular formation (PPRF) in head-restrained monkeys evokes a constant velocity eye movement. Since the PPRF receives significant projections from structures that control coordinated eye-head movements, we asked whether stimulation of the pontine reticular formation in the head-unrestrained animal generates a combined eye-head movement or only an eye movement. Microstimulation of most sites yielded a constant-velocity gaze shift executed as a coordinated eye-head movement, although eye-only movements were evoked from some sites. The eye and head contributions to the stimulation-evoked movements varied across stimulation sites and were drastically different from the lawful relationship observed for visually-guided gaze shifts. These results indicate that the microstimulation activated elements that issued movement commands to the extraocular and, for most sites, neck motoneurons. In addition, the stimulation-evoked changes in gaze were similar in the head-restrained and head-unrestrained conditions despite the assortment of eye and head contributions, suggesting that the vestibulo-ocular reflex (VOR) gain must be near unity during the coordinated eye-head movements evoked by stimulation of the PPRF. These findings contrast the attenuation of VOR gain associated with visually-guided gaze shifts and suggest that the vestibulo-ocular pathway processes volitional and PPRF stimulation-evoked gaze shifts differently.
Figures
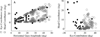





References
-
- Andre-Deshays C, Berthoz A, Revel M. Eye-head coupling in humans. I. Simultaneous recording of isolated motor units in dorsal neck muscles and horizontal eye movements. Exp Brain Res. 1988;69:399–406. - PubMed
-
- Breznen B, Lu SM, Gnadt JW. Analysis of the step response of the saccadic feedback: system behavior. Exp Brain Res. 1996;111:337–344. - PubMed
-
- Chen LL, Walton MM. Head movement evoked by electrical stimulation in the supplementary eye field of the rhesus monkey. J Neurophysiol. 2005;94:4502–4519. - PubMed
-
- Cohen B, Komatsuzaki A. Eye movements induced by stimulation of the pontine reticular formation: evidence for integration in oculomotor pathways. Exp Neurol. 1972;36:101–117. - PubMed
-
- Corneil BD, Olivier E, Munoz DP. Neck muscle activity evoked by stimulation of the monkey superior colliculus. I. Topography and manipulation of stimulation parameters. J Neurophysiol. 2002;88:1980–1999. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

