Intestinal absorption mechanisms of prenylated flavonoids present in the heat-processed Epimedium koreanum Nakai (Yin Yanghuo)
- PMID: 18459036
- PMCID: PMC2574979
- DOI: 10.1007/s11095-008-9602-7
Intestinal absorption mechanisms of prenylated flavonoids present in the heat-processed Epimedium koreanum Nakai (Yin Yanghuo)
Abstract
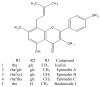
Purpose: The purpose is to determine absorption mechanism of five bioactive prenylated flavonoids (baohuoside I, icariin, epimedine A, B, and C) present in heat-processed Epimedium koreanum Nakai (Yin Yanghuo).
Methods: Transport of five prenylated flavonoids present in heat-processed herbs were studied in the human intestinal Caco-2 model and the perfused rat intestinal model.
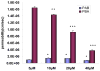
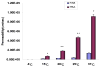
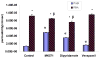
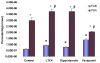
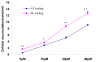
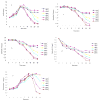
Results: In the perfused rat intestinal model, prenylated flavonoids with a monoglucosidic bond (e.g., icariin) was rapidly hydrolyzed into corresponding metabolites (e.g., baohuoside I). In the Caco-2 model, apical to basolateral permeability of a monoglycoside baohuoside I (1.46 x 10(-6) cm/sec) was more than 2 folds greater than four prenylated flavonoids with 2 or more sugar moieties (<0.6 x 10(-6) cm/sec). The slow apical to basolateral transport of baohuoside I was the result of efflux. This efflux was carrier-mediated and active since its transport was vectorial, concentration- and temperature-dependent with activation energies greater than 15 kcal/mol. Efflux of baohuoside I was significantly suppressed by inhibitors of BCRP and MRP2, whereas efflux of icariin was significantly inhibited only by p-glycoprotein inhibitor verapamil. Because YHH is often heat-processed for better efficacy, we determined and found the optimal condition for increasing contents of more bioavailable flavonoids (i.e., baohuoside I) to be 160-170 degrees C for 5-7 min.
Conclusions: Poor bioavailability of prenylated flavonoids results from their poor intrinsic permeation and transporter-mediated efflux. Heat processing parameters may be optimized to preserve the herb's bioavailable flavonoids, which help retain and improve its efficacy during processing.
Figures
References
-
- Branca F, Lorenzetti S. Health effects of phytoestrogens. Forum Nutr. 2005:100–111. Medline. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

