Preparation of a matrix type multiple-unit gastro retentive floating drug delivery system for captopril based on gas formation technique: in vitro evaluation
- PMID: 18459051
- PMCID: PMC2976957
- DOI: 10.1208/s12249-008-9090-4
Preparation of a matrix type multiple-unit gastro retentive floating drug delivery system for captopril based on gas formation technique: in vitro evaluation
Abstract
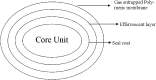
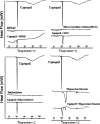
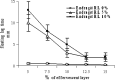
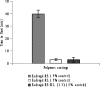
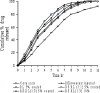
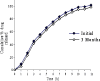
A gastro retentive floating drug delivery system with multiple-unit minitab's based on gas formation technique was developed in order to prolong the gastric residence time and to increase the overall bioavailability of the drug. The system consists of the drug-containing core units prepared by direct compression process, which are coated with three successive layers of an inner seal coat, effervescent layer (sodium bicarbonate) and an outer gas-entrapped polymeric membrane of an polymethacrylates (Eudragit RL30D, RS30D, and combinations of them). Only the system using Eudragit RL30D and combination of them as a gas-entrapped polymeric membrane could float. The time to float decreased as amount of the effervescent agent increased and coating level of gas-entrapped polymeric membrane decreased. The optimum system floated completely within 3 min and maintained the buoyancy over a period of 12 h. The drug release was controlled and linear with the square root of time. Increasing coating level of gas-entrapped polymeric membrane decreased the drug release. Both the rapid floating and the controlled release properties were achieved in the multiple-unit floating drug delivery system developed in this present study. The analysis of the parameter dissolution data after storage at 40 degrees C and 75% RH for 3 months showed, no significant change indicating the two dissolution profiles were considered to be similar (f2 value is more than 50).
Figures

Similar articles
-
Preparation and in vitro evaluation of a multiple-unit floating drug delivery system based on gas formation technique.Int J Pharm. 2006 Nov 6;324(2):136-43. doi: 10.1016/j.ijpharm.2006.06.002. Epub 2006 Jun 9. Int J Pharm. 2006. PMID: 16828997
-
Design and evaluation of polymeric coated minitablets as multiple unit gastroretentive floating drug delivery systems for furosemide.J Pharm Sci. 2009 Jun;98(6):2122-32. doi: 10.1002/jps.21562. J Pharm Sci. 2009. PMID: 19009598
-
Development and in vivo floating behavior of verapamil HCl intragastric floating tablets.AAPS PharmSciTech. 2009;10(1):310-5. doi: 10.1208/s12249-009-9210-9. Epub 2009 Mar 19. AAPS PharmSciTech. 2009. PMID: 19296224 Free PMC article.
-
Polymeric nanofibers: targeted gastro-retentive drug delivery systems.J Drug Target. 2015 Feb;23(2):109-24. doi: 10.3109/1061186X.2014.965715. Epub 2014 Sep 30. J Drug Target. 2015. PMID: 25268275 Review.
-
[Modern polymers in matrix tablets technology].Polim Med. 2014 Jul-Sep;44(3):189-96. Polim Med. 2014. PMID: 25739125 Review. Polish.
Cited by
-
Features and Facts of a Gastroretentive Drug Delivery System-A Review.Turk J Pharm Sci. 2022 Aug 31;19(4):476-487. doi: 10.4274/tjps.galenos.2021.44959. Turk J Pharm Sci. 2022. PMID: 36047602 Free PMC article.
-
Utilizing 4D Printing to Design Smart Gastroretentive, Esophageal, and Intravesical Drug Delivery Systems.Adv Healthc Mater. 2023 Apr;12(10):e2202631. doi: 10.1002/adhm.202202631. Epub 2023 Jan 18. Adv Healthc Mater. 2023. PMID: 36571721 Free PMC article. Review.
-
Impact of anti-tacking agents on properties of gas-entrapped membrane and effervescent floating tablets.AAPS PharmSciTech. 2014 Dec;15(6):1357-69. doi: 10.1208/s12249-014-0161-4. Epub 2014 Jun 14. AAPS PharmSciTech. 2014. PMID: 24927669 Free PMC article.
-
Formulation of metoclopramide HCl gastroretentive film and in vitro- in silico prediction using Gastroplus® PBPK software.Saudi Pharm J. 2022 Dec;30(12):1816-1824. doi: 10.1016/j.jsps.2022.10.011. Epub 2022 Nov 2. Saudi Pharm J. 2022. PMID: 36601510 Free PMC article.
-
Modulating drug release from gastric-floating microcapsules through spray-coating layers.PLoS One. 2014 Dec 3;9(12):e114284. doi: 10.1371/journal.pone.0114284. eCollection 2014. PLoS One. 2014. PMID: 25470374 Free PMC article.
References
-
- Chueh H. R., Zia H., Rhodes C. T. Optimization of sotalol floating and bioadhesive extended release tablet formulations. Drug Dev. Ind. Pharm. 1995;21:1725–1747. doi: 10.3109/03639049509069261. - DOI
-
- Iannuccelli V., Coppi G., Bernabei M. T., Cameroni R. Air compartment multiple-unit system for prolonged gastric residence. Part I. Formulation study. Int. J. Pharm. 1998;174:47–54. doi: 10.1016/S0378-5173(98)00229-4. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

