Fatal acute intestinal pseudoobstruction in mice
- PMID: 18459715
- PMCID: PMC2654011
Fatal acute intestinal pseudoobstruction in mice
Abstract
Here we describe the epizootiology and pathology of spontaneous, fatal acute intestinal pseudoobstruction that occurred in a mouse colony of 1000 breeding pairs, mainly of the C57Bl/6 strain and free from known pathogenic agents. Most of the mice affected were dams in the second week of lactation. At necropsy, segments of the small intestines were distended with fluid contents. Widespread apoptosis of the villus epithelium of the small intestine and superficial epithelial cells of the large intestine, associated with strong expression of active caspase 3, was a distinctive feature. Necrotic enterocytes, mucosal erosions, and acute mucosal inflammation were prominent in some mice, and morphologic signs of toxemia were generally present. No light microscopic neuronal changes were apparent in the gut, and no etiologic agents were identified. These results indicate that sudden activation of apoptosis in the trophically stimulated gut epithelium during peak lactation was instrumental for the fatal outcome of the condition, but the primary cause of the motility dysfunction of the bowel was not established.
Figures





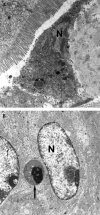
Similar articles
-
Paresis of peristalsis and ileus lead to death in lactating mice.Lab Anim. 1986 Jan;20(1):32-5. doi: 10.1258/002367786781062043. Lab Anim. 1986. PMID: 3754026
-
Atg14 protects the intestinal epithelium from TNF-triggered villus atrophy.Autophagy. 2019 Nov;15(11):1990-2001. doi: 10.1080/15548627.2019.1596495. Epub 2019 Apr 3. Autophagy. 2019. PMID: 30894050 Free PMC article.
-
[Gastro-intestinal lymphocytic and sclerosing leiomyositis (pseudoobstruction) in a dog].Tijdschr Diergeneeskd. 2012 Oct;137(10):666-9. Tijdschr Diergeneeskd. 2012. PMID: 23101332 Dutch.
-
Small Bowel Dysmotility, Pseudoobstruction, and Functional Correlation with Histopathology: Lessons Learned.Curr Gastroenterol Rep. 2020 Feb 20;22(3):14. doi: 10.1007/s11894-020-0748-8. Curr Gastroenterol Rep. 2020. PMID: 32078071 Review.
-
Diagnosis and management of adult patients with chronic intestinal pseudoobstruction.Nutr Clin Pract. 2006 Feb;21(1):16-22. doi: 10.1177/011542650602100116. Nutr Clin Pract. 2006. PMID: 16439766 Review.
Cited by
-
Pathology survey on a captive-bred colony of the Mexican Goodeid, nearly extinct in the wild, Zoogoneticus tequila (Webb & Miller 1998).ScientificWorldJournal. 2013 Oct 30;2013:401468. doi: 10.1155/2013/401468. eCollection 2013. ScientificWorldJournal. 2013. PMID: 24288481 Free PMC article.
-
Toxigenic Profile of Clostridium perfringens Strains Isolated from Natural Ingredient Laboratory Animal Diets.Comp Med. 2022 Feb 1;72(1):50-58. doi: 10.30802/AALAS-CM-22-000013. Epub 2022 Feb 11. Comp Med. 2022. PMID: 35148812 Free PMC article.
-
Adhesive ileus complicating recurrent intestinal pseudo-obstruction in a patient with myasthenia gravis.Case Rep Gastroenterol. 2012 May;6(2):425-8. doi: 10.1159/000339964. Epub 2012 Jun 26. Case Rep Gastroenterol. 2012. PMID: 23055952 Free PMC article.
-
Small Bowel Obstruction in Postpartum Vaginal Delivery due to Prior Abdominal Adhesions Case Report.Case Rep Obstet Gynecol. 2023 Mar 28;2023:6563205. doi: 10.1155/2023/6563205. eCollection 2023. Case Rep Obstet Gynecol. 2023. PMID: 37025389 Free PMC article.
References
-
- Alscher KT, Phang PT, McDonald TE, Walley KR. 2001. Enteral feeding decreases gut apoptosis, permeability, and lung inflammation during murine endotoxemia. Am J Physiol Gastrointest Liver Physiol 281:G569–G576 - PubMed
-
- Botsios DS, Vasiliadis KD. 2003. Factors enhancing intestinal adaptation after bowel compensation. Dig Dis 21:228–236 - PubMed
-
- Brubaker PL, Anini Y. 2003. Direct and indirect mechanisms regulating secretion of glucagon-like peptide 1 and glucagon-like peptide 2. Can J Physiol Pharmacol 81:1005–1012 - PubMed
-
- Cottrell DF, McGorum BC, Pearson GT. 1999. The neurology and enterology of equine grass sickness: a review of basic mechanisms. Neurogastroenterol Motil 11:79–92 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
