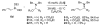Asymmetric petasis reactions catalyzed by chiral biphenols
- PMID: 18459782
- PMCID: PMC2440570
- DOI: 10.1021/ja8018934
Asymmetric petasis reactions catalyzed by chiral biphenols
Abstract
Chiral biphenols catalyze the enantioselective Petasis reaction of alkenyl boronates, secondary amines, and ethyl glyoxylate. The reaction requires the use of 15 mol % of (S)-VAPOL as the catalyst, alkenyl boronates as nucleophiles, ethyl glyoxylate as the aldehyde component, and 3 A molecular sieves as an additive. The chiral alpha-amino ester products are obtained in good yields (71-92%) and high enantiomeric ratios (89:11-98:2). Mechanistic investigations indicate single ligand exchange of acyclic boronate with VAPOL and tetracoordinate boronate intermediates.
Figures
Similar articles
-
Asymmetric Petasis Borono-Mannich Allylation Reactions Catalyzed by Chiral Biphenols.Angew Chem Int Ed Engl. 2017 Feb 1;56(6):1544-1548. doi: 10.1002/anie.201611332. Epub 2017 Jan 4. Angew Chem Int Ed Engl. 2017. PMID: 28052567 Free PMC article.
-
Enantioselective Multicomponent Condensation Reactions of Phenols, Aldehydes, and Boronates Catalyzed by Chiral Biphenols.Org Lett. 2015 Dec 4;17(23):5812-5. doi: 10.1021/acs.orglett.5b02954. Epub 2015 Nov 18. Org Lett. 2015. PMID: 26576776 Free PMC article.
-
Enantioselective addition of boronates to o-quinone methides catalyzed by chiral biphenols.J Am Chem Soc. 2012 Dec 12;134(49):19965-8. doi: 10.1021/ja309076g. Epub 2012 Dec 3. J Am Chem Soc. 2012. PMID: 23206197 Free PMC article.
-
Petasis three-component reactions for the synthesis of diverse heterocyclic scaffolds.Drug Discov Today Technol. 2018 Nov;29:27-33. doi: 10.1016/j.ddtec.2018.06.010. Epub 2018 Jul 17. Drug Discov Today Technol. 2018. PMID: 30471670 Review.
-
Boronic acids and esters in the Petasis-borono Mannich multicomponent reaction.Chem Rev. 2010 Oct 13;110(10):6169-93. doi: 10.1021/cr100108k. Chem Rev. 2010. PMID: 20677749 Review. No abstract available.
Cited by
-
Preparation and facile addition reactions of iminium salts derived from amino ketene silyl acetal and amino silyl enol ether.RSC Adv. 2020 Jul 24;10(46):27874-27883. doi: 10.1039/d0ra05768a. eCollection 2020 Jul 21. RSC Adv. 2020. PMID: 35516926 Free PMC article.
-
Palladium-catalyzed asymmetric three-component reaction between glyoxylic acid, sulfonamides and arylboronic acids for the synthesis of α-arylglycine derivatives.Front Chem. 2023 Mar 13;11:1165618. doi: 10.3389/fchem.2023.1165618. eCollection 2023. Front Chem. 2023. PMID: 36993813 Free PMC article.
-
The Petasis Reaction: Applications and Organic Synthesis-A Comprehensive Review.Top Curr Chem (Cham). 2025 Jan 25;383(1):7. doi: 10.1007/s41061-025-00491-2. Top Curr Chem (Cham). 2025. PMID: 39856385 Review.
-
Asymmetric propargylation of ketones using allenylboronates catalyzed by chiral biphenols.Org Lett. 2011 Aug 5;13(15):4020-3. doi: 10.1021/ol201535b. Epub 2011 Jul 6. Org Lett. 2011. PMID: 21732609 Free PMC article.
-
Recent Trends in the Petasis Reaction: A Review of Novel Catalytic Synthetic Approaches with Applications of the Petasis Reaction.Molecules. 2023 Dec 10;28(24):8032. doi: 10.3390/molecules28248032. Molecules. 2023. PMID: 38138522 Free PMC article. Review.
References
-
- Petasis NA, Akritopoulou I. Tetrahedron Lett. 1993;34:583–586.
- Petasis NA, Zavialov IA. J Am Chem Soc. 1997;119:445–446.
- Petasis NA, Goodman A, Zavialov IA. Tetrahedron. 1997;53:16463–16470.
- Petasis NA, Zavialov IA. J Am Chem Soc. 1998;120:11798–11799.
- Petasis NA. Aust J Chem. 2007;60:795–798.
-
- Harwood LM, Currie GS, Drew MGB, Luke RWA. Chem Commun. 1996:1953–1954.
- Koolmeister T, Södergren M, Scobie M. Tetrahedron Lett. 2002;43:5969–5970.
- Nanda KK, Trotter BW. Tetrahedron Lett. 2005;46:2025–2028.
- Southwood TJ, Curry MC, Hutton CA. Tetrahedron. 2006;62:236–242.
-
- Yamaoka Y, Miyabe H, Takemoto Y. J Am Chem Soc. 2007;129:6686–6687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources