Effect of intestinal microbiota on the induction of regulatory CD25+ CD4+ T cells
- PMID: 18460018
- PMCID: PMC2432106
- DOI: 10.1111/j.1365-2249.2008.03668.x
Effect of intestinal microbiota on the induction of regulatory CD25+ CD4+ T cells
Abstract
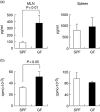
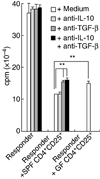
When oral tolerance was induced in either specific pathogen-free (SPF) or germ-free (GF) mice, ovalbumin (OVA) feeding before immunization induced oral tolerance successfully in SPF mice. On the other hand, OVA-specific immunoglobulin G1 (IgG1) and IgE titres in OVA-fed GF mice were comparable to those in phosphate-buffered saline-fed GF mice, thus demonstrating that oral tolerance could not be induced in GF mice. The frequencies of CD25(+) CD4(+)/CD4(+) cells in the mesenteric lymph node (MLN) and the absolute number of CD25(+) CD4(+) cells in the Peyer's patches and MLN of naive GF mice were significantly lower than those in naive SPF mice. In an in vitro assay, the CD25(+) CD4(+) cells from the naive SPF mice suppressed more effectively the proliferation of responder cells in a dose-dependent manner than those from the GF mice. In addition, the CD25(+) CD4(+) regulatory T (T(reg)) cells from the naive SPF mice produced higher amounts of interleukin (IL)-10 and transforming growth factor (TGF)-beta than those from the GF mice. When anti-TGF-beta neutralizing antibody, but not anti-IL-10 neutralizing antibody, was added to the in vitro proliferation assay, the suppressive effect of the CD25(+) CD4(+) T(reg) cells from the SPF mice was attenuated to the same level as that of the CD25(+) CD4(+) cells from the GF mice. In conclusion, the TGF-beta-producing CD25(+) CD4(+) T(reg) cells from the MLN of SPF mice played a major role in oral tolerance induction. In addition, as the regulatory function of the CD25(+) CD4(+) cells from the naive GF mice was much lower than that of the CD25(+) CD4(+) T(reg) cells from the SPF mice, indigenous microbiota are thus considered to contribute to the induction and maintenance of CD25(+) CD4(+) T(reg) cells.
Figures
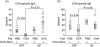

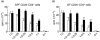
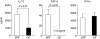
Similar articles
-
Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3-CD25- CD4+ regulatory T cells.J Immunol. 2006 Dec 1;177(11):7634-44. doi: 10.4049/jimmunol.177.11.7634. J Immunol. 2006. PMID: 17114433
-
Sublingual tolerance induction with antigen conjugated to cholera toxin B subunit induces Foxp3+CD25+CD4+ regulatory T cells and suppresses delayed-type hypersensitivity reactions.Scand J Immunol. 2006 Sep;64(3):251-9. doi: 10.1111/j.1365-3083.2006.01823.x. Scand J Immunol. 2006. PMID: 16918694
-
Induction of ovalbumin-specific tolerance by oral administration of Lactococcus lactis secreting ovalbumin.Gastroenterology. 2007 Aug;133(2):517-28. doi: 10.1053/j.gastro.2007.04.073. Epub 2007 May 3. Gastroenterology. 2007. PMID: 17681173
-
The battle against immunopathology: infectious tolerance mediated by regulatory T cells.Cell Mol Life Sci. 2012 Jun;69(12):1997-2008. doi: 10.1007/s00018-011-0907-z. Epub 2011 Dec 29. Cell Mol Life Sci. 2012. PMID: 22205213 Free PMC article. Review.
-
Regulatory T cells and immune tolerance in the intestine.Cold Spring Harb Perspect Biol. 2013 Jul 1;5(7):a018341. doi: 10.1101/cshperspect.a018341. Cold Spring Harb Perspect Biol. 2013. PMID: 23818502 Free PMC article. Review.
Cited by
-
Short-Term Amoxicillin-Induced Perturbation of the Gut Microbiota Promotes Acute Intestinal Immune Regulation in Brown Norway Rats.Front Microbiol. 2020 Mar 26;11:496. doi: 10.3389/fmicb.2020.00496. eCollection 2020. Front Microbiol. 2020. PMID: 32292395 Free PMC article.
-
Regulatory T-cell stability and plasticity in mucosal and systemic immune systems.Mucosal Immunol. 2010 Sep;3(5):443-9. doi: 10.1038/mi.2010.27. Epub 2010 May 26. Mucosal Immunol. 2010. PMID: 20505662 Free PMC article. Review.
-
Intestinal bacteria and the regulation of immune cell homeostasis.Annu Rev Immunol. 2010;28:623-67. doi: 10.1146/annurev-immunol-030409-101330. Annu Rev Immunol. 2010. PMID: 20192812 Free PMC article. Review.
-
The intestinal microbiota in health and disease: the influence of microbial products on immune cell homeostasis.Curr Opin Gastroenterol. 2009 Nov;25(6):496-502. doi: 10.1097/MOG.0b013e328331b6b4. Curr Opin Gastroenterol. 2009. PMID: 19770652 Free PMC article. Review.
-
Long-term repeated daily use of intragastric gavage hinders induction of oral tolerance to ovalbumin in mice.Comp Med. 2014 Oct;64(5):369-76. Comp Med. 2014. PMID: 25402177 Free PMC article.
References
-
- Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. - PubMed
-
- Tsuji NM. Antigen-specific CD4(+) regulatory T cells in the intestine. Inflamm Allergy Drug Targets. 2006;5:191–201. - PubMed
-
- Faria AM, Weiner HL. Oral tolerance and TGF-beta-producing cells. Inflamm Allergy Drug Targets. 2006;5:179–90. - PubMed
-
- Chung Y, Lee SH, Kim DH, Kang CY. Complementary role of CD4+CD25+ regulatory T cells and TGF-beta in oral tolerance. J Leukoc Biol. 2005;77:906–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

