Impact of noncompliance with alendronate and risedronate on the incidence of nonvertebral osteoporotic fractures in elderly women
- PMID: 18460036
- PMCID: PMC2485250
- DOI: 10.1111/j.1365-2125.2008.03178.x
Impact of noncompliance with alendronate and risedronate on the incidence of nonvertebral osteoporotic fractures in elderly women
Abstract
Aims: To evaluate the association between noncompliance with alendronate and risedronate and the risk of nonvertebral osteoporotic fracture in community-dwelling elderly women.
Methods: A nested case-control study was conducted using the Quebec administrative health databases. To be included in the cohort, women needed to be aged > or = 68 years and to have initiated treatment with alendronate or risedronate between 1 January 2002 and 31 March 2005. Cases consisted of all women with an incident nonvertebral osteoporotic fracture occurring > or = 1 year after initiation of therapy. Each case was matched with up to 20 controls using incidence density sampling, according to age (+/- 1 year) and follow-up duration. A woman was noncompliant if she had a medication possession ratio (MPR) <80% for total follow-up duration. Rate ratios (RR) for fracture were estimated through conditional logistic regression analysis, adjusting for potential confounders.
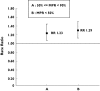
Results: Among the 30 259 women included in the cohort, 1036 nonvertebral fracture cases were identified and were matched to 20 069 controls. Compared with women with a MPR > or = 80%, those with a MPR < 80% had a greater risk of nonvertebral fracture [adjusted RR 1.27, 95% confidence interval (CI) 1.12, 1.44]. Considering hip fracture only, the multivariate model yielded similar results, (adjusted RR 1.28, 95% CI 1.02, 1.61).
Conclusions: Among community-dwelling elderly women, noncompliance with alendronate or risedronate is associated with an increased risk of nonvertebral fracture.
Figures
References
-
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33. - PubMed
-
- Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C. Meta-analyses of therapies for postmenopausal osteoporosis. IX: summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev. 2002;23:570–8. - PubMed
-
- Harrington JT, Ste-Marie LG, Brandi ML, Civitelli R, Fardellone P, Grauer A, Barton I, Boonen S. Risedronate rapidly reduces the risk for nonvertebral fractures in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;74:129–35. - PubMed
-
- Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–8. - PubMed
-
- Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

