A phase 1 trial of nebulised heparin in acute lung injury
- PMID: 18460218
- PMCID: PMC2481447
- DOI: 10.1186/cc6894
A phase 1 trial of nebulised heparin in acute lung injury
Abstract
Introduction: Animal studies of acute lung injury (ALI) suggest nebulised heparin may limit damage from fibrin deposition in the alveolar space and microcirculation. No human studies have been undertaken to date. We assessed the feasibility, safety and potential anticoagulant effects of administration of nebulised heparin to patients with ALI.
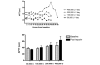
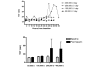
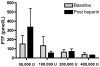
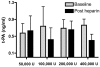
Methods: An open label phase 1 trial of four escalating doses of nebulised heparin was performed. A total of 16 ventilated patients with ALI were studied. The first group was administered a total of 50,000 U/day, the second group 100,000 U/day, the third group 200,000 U/day and the fourth group 400,000 U/day. Assessments of lung function included the PaO2/FiO2 ratio, lung compliance and the alveolar dead space fraction. Monitoring of anticoagulation included the activated partial thromboplastin time (APTT) and the thrombin clotting time. Bronchoalveolar lavage fluid was collected and the prothrombin fragment and tissue plasminogen activator levels were assessed. Analysis of variance was used to compare the effects of dose.
Results: No serious adverse events occurred for any dose. The changes over time for the PaO2/FiO2 ratio, lung compliance and the alveolar dead space fraction levels were similar for all doses. A trend to increased APTT and thrombin clotting time levels was present with higher doses (P = 0.09 and P = 0.1, respectively). For the highest dose, the APTT reached 64 seconds; following cessation of nebulised heparin, the APTT fell to 39 seconds (P = 0.06). In bronchoalveolar lavage samples a trend to reduced prothrombin fragment levels was present with higher doses (P = 0.1), while tissue plasminogen activator levels were similar for all doses.
Conclusion: Administration of nebulised heparin to mechanically ventilated patients with ALI is feasible. Nebulised heparin was not associated with any serious adverse events, and at higher doses it increased APTT levels. Larger trials are required to further investigate the safety and efficacy of nebulised heparin. In these trials due consideration must be given to systemic anticoagulant effects.
Trial registration: Australian Clinical trials registry ACTRN12606000388516.
Figures


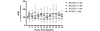
Comment in
-
Nebulised heparin: a new approach to the treatment of acute lung injury?Crit Care. 2008;12(4):170. doi: 10.1186/cc6947. Epub 2008 Jul 25. Crit Care. 2008. PMID: 18671836 Free PMC article.
-
Nebulized heparin reduces levels of pulmonary coagulation activation in acute lung injury.Crit Care. 2010;14(5):445. doi: 10.1186/cc9269. Epub 2010 Oct 20. Crit Care. 2010. PMID: 21067553 Free PMC article. No abstract available.
References
-
- Bersten AD, Edibam C, Hunt T, Moran J. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med. 2002;165:443–448. - PubMed
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. - PubMed
-
- Blaisdell FW. Pathophysiology of the respiratory distress syndrome. Arch Surg. 1974;108:44–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

