Developmental model of static allometry in holometabolous insects
- PMID: 18460425
- PMCID: PMC2593922
- DOI: 10.1098/rspb.2008.0227
Developmental model of static allometry in holometabolous insects
Abstract
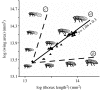
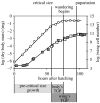
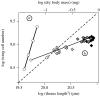
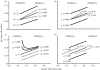
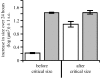
The regulation of static allometry is a fundamental developmental process, yet little is understood of the mechanisms that ensure organs scale correctly across a range of body sizes. Recent studies have revealed the physiological and genetic mechanisms that control nutritional variation in the final body and organ size in holometabolous insects. The implications these mechanisms have for the regulation of static allometry is, however, unknown. Here, we formulate a mathematical description of the nutritional control of body and organ size in Drosophila melanogaster and use it to explore how the developmental regulators of size influence static allometry. The model suggests that the slope of nutritional static allometries, the 'allometric coefficient', is controlled by the relative sensitivity of an organ's growth rate to changes in nutrition, and the relative duration of development when nutrition affects an organ's final size. The model also predicts that, in order to maintain correct scaling, sensitivity to changes in nutrition varies among organs, and within organs through time. We present experimental data that support these predictions. By revealing how specific physiological and genetic regulators of size influence allometry, the model serves to identify developmental processes upon which evolution may act to alter scaling relationships.
Figures

References
-
- Ashburner M. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. Drosophila: a laboratory handbook.
-
- Baehrecke E.H. Ecdysone signaling cascade and regulation of Drosophila metamorphosis. Arch. Insect Biochem. Physiol. 1996;33:231–244. doi:10.1002/(SICI)1520-6327(1996)33:3/4<231::AID-ARCH5>3.0.CO;2-V - DOI - PubMed
-
- Bakker K. Feeding period, growth, and pupation in larvae of Drosophila melanogaster. Entomol. Exp. Appl. 1959;2:171–186. doi:10.1007/BF00302537 - DOI
-
- Basler K, Hafen E. Dynamics of Drosophila eye development and temporal requirements of sevenless expression. Development. 1989;107:723–731. - PubMed
-
- Beadle G, Tatum E, Clancy C. Food level in relation to rate of development and eye pigmentation in Drosophila melanogaster. Biol. Bull. 1938;75:447–462. doi:10.2307/1537573 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
