A soluble activin type IIA receptor induces bone formation and improves skeletal integrity
- PMID: 18460605
- PMCID: PMC2383948
- DOI: 10.1073/pnas.0711263105
A soluble activin type IIA receptor induces bone formation and improves skeletal integrity
Abstract
Diseases that affect the regulation of bone turnover can lead to skeletal fragility and increased fracture risk. Members of the TGF-beta superfamily have been shown to be involved in the regulation of bone mass. Activin A, a TGF-beta signaling ligand, is present at high levels in bone and may play a role in the regulation of bone metabolism. Here we demonstrate that pharmacological blockade of ligand signaling through the high affinity receptor for activin, type II activin receptor (ActRIIA), by administration of the soluble extracellular domain of ActRIIA fused to a murine IgG2a-Fc, increases bone formation, bone mass, and bone strength in normal mice and in ovariectomized mice with established bone loss. These observations support the development of this pharmacological strategy for the treatment of diseases with skeletal fragility.
Conflict of interest statement
Conflict of interest statement: R.S.P., M.C.-B., K.W.U., B.H., J.U., R.K., E.P., A.G., E.D.W., and J.S. are full-time employees of Acceleron Pharma.
Figures

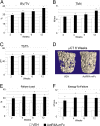
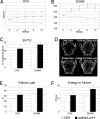
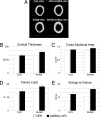
References
-
- Rodan GA. Bone mass homeostasis and bisphosphonate action. Bone. 1997;20:1–4. - PubMed
-
- Lambrinoudaki I, Christodoulakos G, Botsis D. Bisphosphonates. Ann NY Acad Sci. 2006;1092:397–402. - PubMed
-
- Black DM, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. - PubMed
-
- MacDonald BR, Gowen M. Emerging therapies in osteoporosis. Best Pract Res Clin Rheumatol. 2001;15:483–496. - PubMed
-
- Burr D. Microdamage and bone strength. Osteoporosis Int. 2003;14(Suppl 5):67–72. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

