N,N'-Alkane-diyl-bis-3-picoliniums as nicotinic receptor antagonists: inhibition of nicotine-evoked dopamine release and hyperactivity
- PMID: 18460644
- PMCID: PMC3089982
- DOI: 10.1124/jpet.108.136630
N,N'-Alkane-diyl-bis-3-picoliniums as nicotinic receptor antagonists: inhibition of nicotine-evoked dopamine release and hyperactivity
Abstract
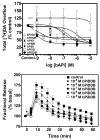
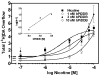
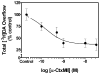
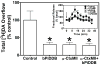
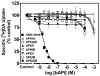
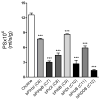
The current study evaluated a new series of N,N'-alkane-diyl-bis-3-picolinium (bAPi) analogs with C6-C12 methylene linkers as nicotinic acetylcholine receptor (nAChR) antagonists, for nicotine-evoked [3H]dopamine (DA) overflow, for blood-brain barrier choline transporter affinity, and for attenuation of discriminative stimulus and locomotor stimulant effects of nicotine. bAPi analogs exhibited little affinity for alpha4beta2* (* indicates putative nAChR subtype assignment) and alpha7* high-affinity ligand binding sites and exhibited no inhibition of DA transporter function. With the exception of C6, all analogs inhibited nicotine-evoked [3H]DA overflow (IC50 = 2 nM-6 microM; Imax = 54-64%), with N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB; C12) being most potent. bPiDDB did not inhibit electrically evoked [3H]DA overflow, suggesting specific nAChR inhibitory effects and a lack of toxicity to DA neurons. Schild analysis suggested that bPiDDB interacts in an orthosteric manner at nAChRs mediating nicotine-evoked [3H]DA overflow. To determine whether bPiDDB interacts with alpha-conotoxin MII-sensitive alpha6beta2-containing nAChRs, slices were exposed concomitantly to maximally effective concentrations of bPiDDB (10 nM) and alpha-conotoxin MII (1 nM). Inhibition of nicotine-evoked [3H]DA overflow was not different with the combination compared with either antagonist alone, suggesting that bPiDDB interacts with alpha6beta2-containing nAChRs. C7, C8, C10, and C12 analogs exhibited high affinity for the blood-brain barrier choline transporter in vivo, suggesting brain bioavailability. Although none of the analogs altered the discriminative stimulus effect of nicotine, C8, C9, C10, and C12 analogs decreased nicotine-induced hyperactivity in nicotine-sensitized rats, without reducing spontaneous activity. Further development of nAChR antagonists that inhibit nicotine-evoked DA release and penetrate brain to antagonize DA-mediated locomotor stimulant effects of nicotine as novel treatments for nicotine addiction is warranted.
Figures





Similar articles
-
The novel nicotinic receptor antagonist, N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB), inhibits nicotine-evoked [(3)H]norepinephrine overflow from rat hippocampal slices.Biochem Pharmacol. 2009 Oct 1;78(7):889-97. doi: 10.1016/j.bcp.2009.07.010. Epub 2009 Jul 23. Biochem Pharmacol. 2009. PMID: 19631612 Free PMC article.
-
bPiDI: a novel selective α6β2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse.Br J Pharmacol. 2011 May;163(2):346-57. doi: 10.1111/j.1476-5381.2011.01220.x. Br J Pharmacol. 2011. PMID: 21232049 Free PMC article.
-
r-bPiDI, an α6β2* Nicotinic Receptor Antagonist, Decreases Nicotine-Evoked Dopamine Release and Nicotine Reinforcement.Neurochem Res. 2015 Oct;40(10):2121-30. doi: 10.1007/s11064-015-1680-4. Epub 2015 Jul 31. Neurochem Res. 2015. PMID: 26227997 Free PMC article.
-
Nicotinic receptor antagonists as treatments for nicotine abuse.Adv Pharmacol. 2014;69:513-51. doi: 10.1016/B978-0-12-420118-7.00013-5. Adv Pharmacol. 2014. PMID: 24484986 Free PMC article. Review.
-
Desensitization of nicotinic acetylcholine receptors as a strategy for drug development.J Pharmacol Exp Ther. 2009 Feb;328(2):364-70. doi: 10.1124/jpet.108.145292. Epub 2008 Nov 20. J Pharmacol Exp Ther. 2009. PMID: 19023041 Free PMC article. Review.
Cited by
-
Selective inhibition of acetylcholine-evoked responses of alpha7 neuronal nicotinic acetylcholine receptors by novel tris- and tetrakis-azaaromatic quaternary ammonium antagonists.Mol Pharmacol. 2009 Sep;76(3):652-66. doi: 10.1124/mol.109.056176. Epub 2009 Jun 25. Mol Pharmacol. 2009. PMID: 19556356 Free PMC article.
-
Pharmacologically distinct nicotinic acetylcholine receptors drive efferent-mediated excitation in calyx-bearing vestibular afferents.J Neurosci. 2015 Feb 25;35(8):3625-43. doi: 10.1523/JNEUROSCI.3388-14.2015. J Neurosci. 2015. PMID: 25716861 Free PMC article.
-
Bis-azaaromatic quaternary ammonium salts as ligands for the blood-brain barrier choline transporter.Bioorg Med Chem Lett. 2010 Jun 1;20(11):3208-10. doi: 10.1016/j.bmcl.2010.04.098. Epub 2010 Apr 24. Bioorg Med Chem Lett. 2010. PMID: 20462759 Free PMC article.
-
The novel nicotinic receptor antagonist, N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB), inhibits nicotine-evoked [(3)H]norepinephrine overflow from rat hippocampal slices.Biochem Pharmacol. 2009 Oct 1;78(7):889-97. doi: 10.1016/j.bcp.2009.07.010. Epub 2009 Jul 23. Biochem Pharmacol. 2009. PMID: 19631612 Free PMC article.
-
bPiDI: a novel selective α6β2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse.Br J Pharmacol. 2011 May;163(2):346-57. doi: 10.1111/j.1476-5381.2011.01220.x. Br J Pharmacol. 2011. PMID: 21232049 Free PMC article.
References
-
- Allen DD, Lockman PR, Roder KE, Dwoskin LP, Crooks PA. Active transport of high-affinity choline and nicotine analogs into the central nervous system by the blood-brain barrier choline transporter. J Pharmacol Exp Ther. 2003;304:1268–74. - PubMed
-
- Azam L, Winzer-Serhan UH, Chen YL, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. - PubMed
-
- Boye SM, Grant RJ, Clarke PB. Disruption of dopaminergic neurotransmission in nucleus accumbens core inhibits the locomotor stimulant effects of nicotine and D-amphetamine in rats. Neuropharmacology. 2001;40:792–805. - PubMed
-
- Cao Y, Surowy CS, Puttfarcken PS. Different nicotinic acetylcholine receptor subtypes mediating striatal and prefrontal cortical [3H]dopamine release. Neuropharm. 2005;48:72–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

