Complex dynamics of osteoclast formation and death in long-term cultures
- PMID: 18461134
- PMCID: PMC2330067
- DOI: 10.1371/journal.pone.0002104
Complex dynamics of osteoclast formation and death in long-term cultures
Abstract
Background: Osteoclasts, cells responsible for bone resorption, contribute to the development of degenerative, metabolic and neoplastic bone diseases, which are often characterized by persistent changes in bone microenvironment. We aimed to investigate the dynamics of osteoclast formation and death in cultures that considerably exceeded the length of standard protocol and to design a mathematical model describing osteoclastogenesis.
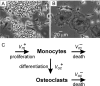
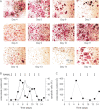
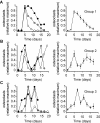
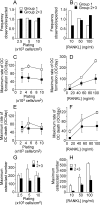
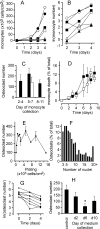
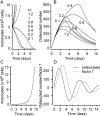
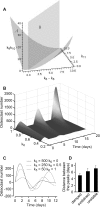
Methodology/principal findings: RAW 264.7 monocytic cells fuse to form multinucleated osteoclasts upon treatment with pro-resorptive cytokine RANKL. We have found that in long-term experiments (15-26 days), the dynamics of changes in osteoclast numbers was remarkably complex and qualitatively variable in different experiments. Whereas 19 of 46 experiments exhibited single peak of osteoclast formation, in 27 experiments we observed development of successive waves of osteoclast formation and death. Periodic changes in osteoclast numbers were confirmed in long-term cultures of mouse bone marrow cells treated with M-CSF and RANKL. Because the dynamics of changes in osteoclast numbers was found to be largely independent of monocytes, a two-species model of ordinary differential equations describing the changes in osteoclasts and monocytes was ineffective in recapitulating the oscillations in osteoclast numbers. Following experimental observation that medium collected from mature osteoclasts inhibited osteoclastogenesis in fresh cultures, we introduced a third variable, factor f, to describe osteoclast-derived inhibitor. This model allowed us to simulate the oscillatory changes in osteoclasts, which were coupled to oscillatory changes in the factor f, whereas monocytes changed exponentially. Importantly, to achieve the experimentally observed oscillations with increasing amplitude, we also had to assume that osteoclast presence stimulates osteoclast formation.
Conclusions/significance: This study identifies the critical role for osteoclast autocrine regulation in controlling long-term dynamic of osteoclast formation and death and describes the complementary roles for negative and positive feedback mediators in determining the sharp dynamics of activation and inactivation of osteoclasts.
Conflict of interest statement
Figures
References
-
- Stern PH. Antiresorptive agents and osteoclast apoptosis. J Cell Biochem. 2007;101:1087–1096. - PubMed
-
- Grey A. Emerging pharmacologic therapies for osteoporosis. Expert Opin Emerg Drugs. 2007;12:493–508. - PubMed
-
- Karsdal MA, Martin TJ, Bollerslev J, Christiansen C, Henriksen K. Are nonresorbing osteoclasts sources of bone anabolic activity? J Bone Miner Res. 2007;22:487–494. - PubMed
-
- Lawson J. Drug-induced metabolic bone disorders. Semin Musculoskelet Radiol. 2002;6:285–297. - PubMed
-
- Takahashi N, Udagawa N, Kobayashi Y, Suda T. Generation of osteoclasts in vitro, and assay of osteoclast activity. Methods Mol Med. 2007;135:285–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

