Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions
- PMID: 18461168
- PMCID: PMC2346555
- DOI: 10.1371/journal.pone.0002122
Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions
Abstract
Background: Human embryonic stem cells (hESC) provide a unique model to study early events in human development. The hESC-derived cells can potentially be used to replace or restore different tissues including neuronal that have been damaged by disease or injury.
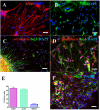
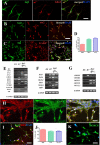
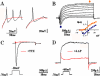
Methodology and principal findings: The cells of two different hESC lines were converted to neural rosettes using adherent and chemically defined conditions. The progenitor cells were exposed to retinoic acid (RA) or to human recombinant basic fibroblast growth factor (bFGF) in the late phase of the rosette formation. Exposing the progenitor cells to RA suppressed differentiation to rostral forebrain dopamine neural lineage and promoted that of spinal neural tissue including motor neurons. The functional characteristics of these differentiated neuronal precursors under both, rostral (bFGF) and caudalizing (RA) signals were confirmed by patch clamp analysis.
Conclusions/significance: These findings suggest that our differentiation protocol has the capacity to generate region-specific and electrophysiologically active neurons under in vitro conditions without embryoid body formation, co-culture with stromal cells and without presence of cells of mesodermal or endodermal lineages.
Conflict of interest statement
Figures



References
-
- Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. - PubMed
-
- Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, et al. Induced neuronal differentiation of human embryonic stem cells. Brain Res. 2001;913:201–205. - PubMed
-
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. - PubMed
-
- Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, et al. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

