SalK/SalR, a two-component signal transduction system, is essential for full virulence of highly invasive Streptococcus suis serotype 2
- PMID: 18461172
- PMCID: PMC2358977
- DOI: 10.1371/journal.pone.0002080
SalK/SalR, a two-component signal transduction system, is essential for full virulence of highly invasive Streptococcus suis serotype 2
Abstract
Background: Streptococcus suis serotype 2 (S. suis 2, SS2) has evolved into a highly infectious entity, which caused the two recent large-scale outbreaks of human SS2 epidemic in China, and is characterized by a toxic shock-like syndrome. However, the molecular pathogenesis of this new emerging pathogen is still poorly understood.
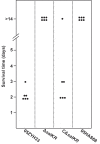
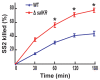
Methodology/principal findings: 89K is a newly predicted pathogenicity island (PAI) which is specific to Chinese epidemic strains isolated from these two SS2 outbreaks. Further bioinformatics analysis revealed a unique two-component signal transduction system (TCSTS) located in the candidate 89K PAI, which is orthologous to the SalK/SalR regulatory system of Streptococcus salivarius. Knockout of salKR eliminated the lethality of SS2 in experimental infection of piglets. Functional complementation of salKR into the isogenic mutant DeltasalKR restored its soaring pathogenicity. Colonization experiments showed that the DeltasalKR mutant could not colonize any susceptible tissue of piglets when administered alone. Bactericidal assays demonstrated that resistance of the mutant to polymorphonuclear leukocyte (PMN)-mediated killing was greatly decreased. Expression microarray analysis exhibited a transcription profile alteration of 26 various genes down-regulated in the DeltasalKR mutant.
Conclusions/significance: These findings suggest that SalK/SalR is requisite for the full virulence of ethnic Chinese isolates of highly pathogenic SS2, thus providing experimental evidence for the validity of this bioinformatically predicted PAI.
Conflict of interest statement
Figures




Similar articles
-
Proteome analysis of the two-component SalK/SalR system in Epidemic Streptococcus suis serotype 2.Curr Microbiol. 2013 Jul;67(1):118-22. doi: 10.1007/s00284-013-0343-4. Epub 2013 Mar 6. Curr Microbiol. 2013. PMID: 23463517
-
Uncovering newly emerging variants of Streptococcus suis, an important zoonotic agent.Trends Microbiol. 2010 Mar;18(3):124-31. doi: 10.1016/j.tim.2009.12.003. Epub 2010 Jan 12. Trends Microbiol. 2010. PMID: 20071175 Review.
-
A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates.PLoS One. 2007 Mar 21;2(3):e315. doi: 10.1371/journal.pone.0000315. PLoS One. 2007. PMID: 17375201 Free PMC article.
-
Virulence genotyping and population analysis of Streptococcus suis serotype 2 isolates from China.Infect Genet Evol. 2015 Dec;36:483-489. doi: 10.1016/j.meegid.2015.08.021. Epub 2015 Aug 21. Infect Genet Evol. 2015. PMID: 26303637
-
Streptococcus suis: a re-emerging pathogen associated with occupational exposure to pigs or pork products. Part II - Pathogenesis.Ann Agric Environ Med. 2018 Mar 14;25(1):186-203. doi: 10.26444/aaem/85651. Epub 2018 Mar 2. Ann Agric Environ Med. 2018. PMID: 29575852 Review.
Cited by
-
The orphan response regulator CovR: a globally negative modulator of virulence in Streptococcus suis serotype 2.J Bacteriol. 2009 Apr;191(8):2601-12. doi: 10.1128/JB.01309-08. Epub 2009 Jan 30. J Bacteriol. 2009. PMID: 19181815 Free PMC article.
-
A functional peptidoglycan hydrolase characterized from T4SS in 89K pathogenicity island of epidemic Streptococcus suis serotype 2.BMC Microbiol. 2014 Mar 22;14:73. doi: 10.1186/1471-2180-14-73. BMC Microbiol. 2014. PMID: 24655418 Free PMC article.
-
The Two-Component Signaling System VraSRss Is Critical for Multidrug Resistance and Full Virulence in Streptococcus suis Serotype 2.Infect Immun. 2018 Jun 21;86(7):e00096-18. doi: 10.1128/IAI.00096-18. Print 2018 Jul. Infect Immun. 2018. PMID: 29685990 Free PMC article.
-
Streptococcus suis DivIVA Protein Is a Substrate of Ser/Thr Kinase STK and Involved in Cell Division Regulation.Front Cell Infect Microbiol. 2018 Mar 20;8:85. doi: 10.3389/fcimb.2018.00085. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29616196 Free PMC article.
-
Streptococcus suis in invasive human infections in Poland: clonality and determinants of virulence and antimicrobial resistance.Eur J Clin Microbiol Infect Dis. 2016 Jun;35(6):917-25. doi: 10.1007/s10096-016-2616-x. Epub 2016 Mar 15. Eur J Clin Microbiol Infect Dis. 2016. PMID: 26980093 Free PMC article.
References
-
- Staats JJ, Feder I, Okwumabua O, Chengappa MM. Streptococcus suis: past and present. Vet Res Commun. 1997;21:381–407. - PubMed
-
- Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis. 2007;7:201–209. - PubMed
-
- Normile D. Infectious diseases. WHO probes deadliness of China's pig-borne disease. Science. 2005;309:1308–1309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

