IL-10-dependent partial refractoriness to Toll-like receptor stimulation modulates gut mucosal dendritic cell function
- PMID: 18461564
- PMCID: PMC2988418
- DOI: 10.1002/eji.200737909
IL-10-dependent partial refractoriness to Toll-like receptor stimulation modulates gut mucosal dendritic cell function
Abstract
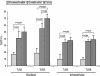
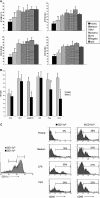
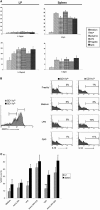
The default response of the intestinal immune system to most antigens is the induction of immunological tolerance, which is difficult to reconcile with the constant exposure to ligands for TLR and other pattern recognition receptors. We showed previously that dendritic cells (DC) from the lamina propria of normal mouse intestine may be inherently tolerogenic and here we have explored how this might relate to the expression and function of Toll-like receptors (TLR). Lamina propria (LP) DC showed higher levels of TLR 2, 3, 4 and 9 protein expression than spleen and MLN DC, with most TLR-expressing DC in the gut being CD11c(lo), class II MHC(lo), CD103(-), CD11b(-) and F4/80(-). TLR expression by lamina propria DC was low in the upper small intestine and higher in distal small intestine and colon. Freshly isolated lamina propria DC expressed some CD40, CD80, CD86 and functional CCR7. These were up-regulated on CD11c(lo), but not on CD11c(hi) LP DC by stimulation via TLR. However, there was little induction of IL-12 by either subset in response to TLR ligation. This was associated with constitutive IL-10 production and was reversed by blocking IL-10 function. Thus, IL-10 may maintain LP DC in a partially unresponsive state to TLR ligation, allowing them to have a critical role in immune homeostasis in the gut.
Figures

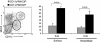


Comment in
-
Lamina propria dendritic cells: for whom the bell TOLLs?Eur J Immunol. 2008 Jun;38(6):1483-6. doi: 10.1002/eji.200838435. Eur J Immunol. 2008. PMID: 18493978
References
-
- Mowat A, Mc I. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003;3:331–341. - PubMed
-
- Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002;20:621–667. - PubMed
-
- Chirdo FG, Millington OR, Beacock-Sharp H, Mowat A, Mc I. Immunomodulatory dendritic cells in intestinal lamina propria. Eur. J. Immunol. 2005;35:1831–1840. - PubMed
-
- Viney JL, Mowat AMcI, O'Malley JM, Williamson E, Fanger N. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J. Immunol. 1998;160:5815–5825. - PubMed
-
- Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K, Guo Z, et al. CCR7 Is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J. Immunol. 2006;176:803–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

