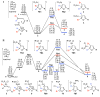
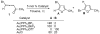Mechanistic insights into the gold-catalyzed cycloisomerization of bromoallenyl ketones: ligand-controlled regioselectivity
- PMID: 18461941
- PMCID: PMC2435085
- DOI: 10.1021/ja802144t
Mechanistic insights into the gold-catalyzed cycloisomerization of bromoallenyl ketones: ligand-controlled regioselectivity
Abstract
Through computational and experimental studies, the mechanisms of gold-catalyzed cycloisomerization of bromoallenyl ketones in toluene have been elucidated. The divergent 1,2-migrations for the Au(I)- and Au(III)-catalyzed reactions have been investigated, and the results confirmed that the regiochemistry is ligand-dependent in cases of Au(PR3)L (L = Cl, OTf, BF4, and SbF6) catalysts.
Figures
References
-
-
For recent reviews, see: Hashmi ASK, Hutchings GJ. Angew Chem Int Ed. 2006;45:7896.Zhang L, Sun J, Kozmin SA. Adv Synth Catal. 2006;348:2271.Gorin DJ, Toste FD. Nature. 2007;446:395.Hashmi ASK. Chem Rev. 2007;107:3180.
-
-
-
For higher oxophilicity of AuCl3 compared to that of AuCl, see: Yamamoto Y. J Org Chem. 2007;72:7817.
-
-
- Hashmi ASK. Angew Chem Int Ed. 2005;44:6990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources