Biogenesis of the trypanosome endo-exocytotic organelle is cytoskeleton mediated
- PMID: 18462016
- PMCID: PMC2365980
- DOI: 10.1371/journal.pbio.0060105
Biogenesis of the trypanosome endo-exocytotic organelle is cytoskeleton mediated
Abstract
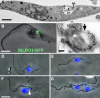
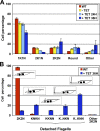
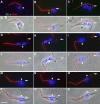
Trypanosoma brucei is a protozoan parasite that is used as a model organism to study such biological phenomena as gene expression, protein trafficking, and cytoskeletal biogenesis. In T. brucei, endocytosis and exocytosis occur exclusively through a sequestered organelle called the flagellar pocket (FP), an invagination of the pellicular membrane. The pocket is the sole site for specific receptors thus maintaining them inaccessible to components of the innate immune system of the mammalian host. The FP is also responsible for the sorting of protective parasite glycoproteins targeted to, or recycling from, the pellicular membrane, and for the removal of host antibodies from the cell surface. Here, we describe the first characterisation of a flagellar pocket cytoskeletal protein, BILBO1. BILBO1 functions to form a cytoskeleton framework upon which the FP is made and which is also required and essential for FP biogenesis and cell survival. Remarkably, RNA interference (RNAi)-mediated ablation of BILBO1 in insect procyclic-form parasites prevents FP biogenesis and induces vesicle accumulation, Golgi swelling, the aberrant repositioning of the new flagellum, and cell death. Cultured bloodstream-form parasites are also nonviable when subjected to BILBO1 RNAi. These results provide the first molecular evidence for cytoskeletally mediated FP biogenesis.
Conflict of interest statement
Figures




References
-
- Landfear SM, Ignatushchenko M. The flagellum and flagellar pocket of trypanosomatids. Mol Biochem Parasitol. 2001;115:1–17. - PubMed
-
- Overath P, Engstler M. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol Microbiol. 2004;53:735–744. - PubMed
-
- De Souza W. Secretory organelles of pathogenic protozoa. An Acad Bras Cienc. 2006;78:271–291. - PubMed
-
- Field MC, Natesan SK, Gabernet-Castello C, Lila Koumandou V. Intracellular trafficking in the trypanosomatids. Traffic. 2007;8:629–639. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

