Role of PKCepsilon in PGF2alpha-stimulated MMP-2 secretion from human ciliary muscle cells
- PMID: 18462068
- PMCID: PMC2981373
- DOI: 10.1089/jop.2008.0014
Role of PKCepsilon in PGF2alpha-stimulated MMP-2 secretion from human ciliary muscle cells
Abstract
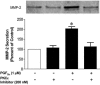
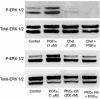
Studies were designed to examine the roles of individual protein kinase C (PKC) isoforms in the prostaglandin F(2alpha) (PGF(2alpha))-induced matrix metalloproteinase-2 (MMP-2) secretion from human ciliary muscle cells. Studies utilized primary cultures of human ciliary muscle cells. Individual PKC isoforms were detected by Western blotting, using PKC-isoform-specific antibodies. To evaluate MMP-2 secretion, cells were serum-starved overnight, treated with PGF(2alpha) (1 micromol/L) for 4 h and the media analyzed for MMP-2 by Western blotting. To assess ERK1/2 activation, cells were serum-starved overnight, treated with PGF(2alpha) (1 micromol/L) for 5 min and cell lysates analyzed for ERK1/2 phosphorylation by Western blot analysis. To evaluate the roles of individual PKC isoforms, cells were pretreated with PKC inhibitors or siRNAs prior to the addition of PGF(2alpha). In cultured human ciliary muscle cells, the PKC isoforms exhibiting the highest level of expression were PKCalpha, epsilon, iota and lambda. The delta and eta isoforms exhibited moderate levels of expression and beta, gamma, and phi were not detected. The administration of PGF(2alpha) (1 micromol/L) primarily induced the translocation of PKCepsilon from cytosol to the membrane fraction, as well as increased MMP-2 secretion and ERK1/2 phosphorylation. The secretion of MMP-2 was inhibited by pretreatment with the broad-range PKC inhibitor, chelerythrine chloride; however, this response was not blocked by Go-6976, an inhibitor of conventional PKC isoforms. The PGF(2alpha)-induced secretion of MMP-2 was also blocked by pretreatment with the PKCepsilon-selective peptide translocation inhibitor, EAVSLKPT, or the transfection of siRNA-targeting PKCepsilon. The activation of ERK1/2 was inhibited by chelerythrine and the PKCepsilon translocation inhibitor. Human ciliary muscle cells express the alpha, epsilon, iota and lambda PKC isoforms. Stimulation of FP receptors in these cells activates PKCepsilon, resulting in ERK1/2 activation and an eventual increase in MMP-2 secretion. These data support the idea that the activation of FP receptors in vivo modulate uveoscleral outflow through the PKCepsilon-dependent secretion of MMPs.
Figures
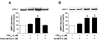

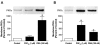

Similar articles
-
Acute effects of PGF2alpha on MMP-2 secretion from human ciliary muscle cells: a PKC- and ERK-dependent process.Invest Ophthalmol Vis Sci. 2005 May;46(5):1706-13. doi: 10.1167/iovs.04-0993. Invest Ophthalmol Vis Sci. 2005. PMID: 15851572
-
Endothelin-1 enhances eicosanoids-induced coronary smooth muscle contraction by activating specific protein kinase C isoforms.Hypertension. 2001 Feb;37(2 Pt 2):497-504. doi: 10.1161/01.hyp.37.2.497. Hypertension. 2001. PMID: 11230325
-
Expression and activation of protein kinase C isozymes by prostaglandin F(2alpha) in the early- and mid-luteal phase bovine corpus luteum.Biol Reprod. 2004 Feb;70(2):379-84. doi: 10.1095/biolreprod.103.020420. Epub 2003 Oct 1. Biol Reprod. 2004. PMID: 14522831
-
Ethanol-Induced Changes in PKCε: From Cell to Behavior.Front Neurosci. 2018 Apr 12;12:244. doi: 10.3389/fnins.2018.00244. eCollection 2018. Front Neurosci. 2018. PMID: 29706864 Free PMC article. Review.
-
The role of protein kinase C epsilon in neural signal transduction and neurogenic diseases.Front Med. 2011 Mar;5(1):70-6. doi: 10.1007/s11684-011-0119-9. Epub 2011 Mar 17. Front Med. 2011. PMID: 21681677 Review.
Cited by
-
The roles of prostaglandin F2 in regulating the expression of matrix metalloproteinase-12 via an insulin growth factor-2-dependent mechanism in sheared chondrocytes.Signal Transduct Target Ther. 2018 Nov 23;3:27. doi: 10.1038/s41392-018-0029-2. eCollection 2018. Signal Transduct Target Ther. 2018. PMID: 30510777 Free PMC article.
-
Heparin binding VEGF isoforms attenuate hyperoxic embryonic lung growth retardation via a FLK1-neuropilin-1-PKC dependent pathway.Respir Res. 2014 Mar 19;15(1):32. doi: 10.1186/1465-9921-15-32. Respir Res. 2014. PMID: 24641672 Free PMC article.
-
A review on the role of L-carnitine in the management of tamoxifen side effects in treated women with breast cancer.Tumour Biol. 2014 Apr;35(4):2845-55. doi: 10.1007/s13277-013-1477-5. Epub 2013 Dec 12. Tumour Biol. 2014. PMID: 24338689 Review.
-
Endothelial dysfunction and oxidative stress in polycystic kidney disease.Am J Physiol Renal Physiol. 2014 Dec 1;307(11):F1198-206. doi: 10.1152/ajprenal.00327.2014. Epub 2014 Sep 18. Am J Physiol Renal Physiol. 2014. PMID: 25234311 Free PMC article. Clinical Trial.
References
-
- Abramovitz M. Boie Y. Nguyen T., et al. Cloning and expression of a cDNA for the human prostanoid FP receptor. J. Biol. Chem. 1994;269:2632–2636. - PubMed
-
- Sugimoto Y. Hasumoto K. Namba T., et al. Cloning and expression of a cDNA for mouse prostaglandin F receptor. J. Biol. Chem. 1994;269:1356–1360. - PubMed
-
- Sakamoto K. Ezashi T. Miwa K., et al. Molecular cloning and expression of a cDNA of the bovine prostaglandin F2-alpha receptor. J. Biol. Chem. 1994;269:3881–3886. - PubMed
-
- Husain S. Kaddour-Djebbar I. Abdel-Latif A.A. Alterations in arachidonic acid release and phospholipase C-beta(1) expression in glaucomatous human ciliary muscle cells. Invest. Ophthalmol. Vis. Sci. 2002;43:1127–1134. - PubMed
-
- Sharif N.A. Crider J.Y. Husain S., et al. Human ciliary muscle cell responses to FP-class prostaglandin analogs: Phosphoinositide hydrolysis, intracellular Ca(2+) mobilization, and MAP-kinase activation. J. Ocul. Pharmacol. Ther. 2003;19:437–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

