Neutrophil surface presentation of the anti-neutrophil cytoplasmic antibody-antigen proteinase 3 depends on N-terminal processing
- PMID: 18462208
- PMCID: PMC2453217
- DOI: 10.1111/j.1365-2249.2008.03663.x
Neutrophil surface presentation of the anti-neutrophil cytoplasmic antibody-antigen proteinase 3 depends on N-terminal processing
Abstract
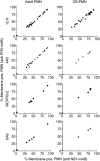
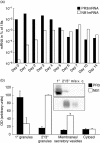
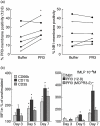
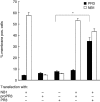
The neutrophil serine protease proteinase 3 (PR3) is a main autoantigen in anti-neutrophil cytoplasmic antibody-associated vasculitis. PR3 surface presentation on neutrophilic granulocytes, the main effector cells, is pathogenically important. PR3 is presented by the NB1 (CD177) glycoprotein, but how the presentation develops during neutrophil differentiation is not known. An N-terminally unprocessed PR3 (proPR3) is produced early during neutrophil development and promotes myeloid cell differentiation. We therefore investigated if PR3 presentation depended on NB1 during neutrophil differentiation and if PR3 and proPR3 could both be presented by NB1. In contrast to mature neutrophils, differentiating neutrophils showed an early NB1-independent PR3 surface display that was recognized by only two of four monoclonal anti-PR3 antibodies and occurred in parallel with proPR3, but not PR3 secretion, suggesting that the NB1-independent surface PR3 was proPR3. PR3 gene expression preceeded NB1. When the NB1 receptor was detected on the surface, a mode of PR3 surface display similar to mature neutrophils developed together with the degranulation system. Ectopic expression studies showed that NB1 was a sufficient receptor for PR3 but not proPR3. ProPR3 display on the plasma membrane may influence the bone marrow microenvironment. NB1-mediated PR3 presentation depended on PR3 N-terminal processing implicating the PR3-N-terminus as NB1-binding site.
Figures


Similar articles
-
Complement receptor Mac-1 is an adaptor for NB1 (CD177)-mediated PR3-ANCA neutrophil activation.J Biol Chem. 2011 Mar 4;286(9):7070-81. doi: 10.1074/jbc.M110.171256. Epub 2010 Dec 30. J Biol Chem. 2011. PMID: 21193407 Free PMC article.
-
A hydrophobic patch on proteinase 3, the target of autoantibodies in Wegener granulomatosis, mediates membrane binding via NB1 receptors.J Biol Chem. 2008 Dec 19;283(51):35976-82. doi: 10.1074/jbc.M806754200. Epub 2008 Oct 14. J Biol Chem. 2008. PMID: 18854317
-
NB1 mediates surface expression of the ANCA antigen proteinase 3 on human neutrophils.Blood. 2007 May 15;109(10):4487-93. doi: 10.1182/blood-2006-10-055327. Epub 2007 Jan 23. Blood. 2007. PMID: 17244676
-
Membrane-bound proteinase 3 and its receptors: relevance for the pathogenesis of Wegener's Granulomatosis.Autoimmun Rev. 2009 May;8(6):510-4. doi: 10.1016/j.autrev.2008.01.003. Epub 2009 Feb 4. Autoimmun Rev. 2009. PMID: 19185066 Review.
-
Pathogenicity of Proteinase 3-Anti-Neutrophil Cytoplasmic Antibody in Granulomatosis With Polyangiitis: Implications as Biomarker and Future Therapies.Front Immunol. 2021 Feb 18;12:571933. doi: 10.3389/fimmu.2021.571933. eCollection 2021. Front Immunol. 2021. PMID: 33679731 Free PMC article. Review.
Cited by
-
Impaired phagocytosis and reactive oxygen species production in phagocytes is associated with systemic vasculitis.Arthritis Res Ther. 2016 Apr 22;18:92. doi: 10.1186/s13075-016-0994-1. Arthritis Res Ther. 2016. PMID: 27102815 Free PMC article.
-
Complement receptor Mac-1 is an adaptor for NB1 (CD177)-mediated PR3-ANCA neutrophil activation.J Biol Chem. 2011 Mar 4;286(9):7070-81. doi: 10.1074/jbc.M110.171256. Epub 2010 Dec 30. J Biol Chem. 2011. PMID: 21193407 Free PMC article.
-
Loss of expression of neutrophil proteinase-3: a factor contributing to thrombotic risk in paroxysmal nocturnal hemoglobinuria.Haematologica. 2011 Jul;96(7):954-62. doi: 10.3324/haematol.2010.029298. Epub 2011 May 5. Haematologica. 2011. PMID: 21546506 Free PMC article.
-
Proteinase 3 expression on the neutrophils of patients with paroxysmal nocturnal hemoglobinuria.Exp Ther Med. 2018 Mar;15(3):2525-2532. doi: 10.3892/etm.2017.5662. Epub 2017 Dec 21. Exp Ther Med. 2018. PMID: 29467851 Free PMC article.
-
Characterization of the CD177 interaction with the ANCA antigen proteinase 3.Sci Rep. 2017 Feb 27;7:43328. doi: 10.1038/srep43328. Sci Rep. 2017. PMID: 28240246 Free PMC article.
References
-
- Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet. 2006;368:404–18. - PubMed
-
- Morgan MD, Harper L, Williams J, Savage C. Anti-neutrophil cytoplasm-associated glomerulonephritis. J Am Soc Nephrol. 2006;17:1224–34. - PubMed
-
- Woywodt A, Streiber F, de Groot K, Regelsberger H, Haller H, Haubitz M. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet. 2003;361:206–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

